Abstract
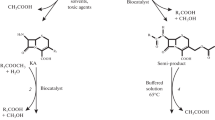
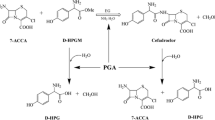
The article deals with experimental determination of ionization constants and solubility for the compounds (target products, initial β-lactams, acylating agents and by-products) involved in enzymatic synthesis of some therapeutically used aminopenicillins and aminocephalosporins, namely ampicillin, amoxicillin, cephalexin, cephadroxil, cephaloglycin, cefaclor, cefprozil, cefatrizine. Methodology of investigations and the evaluation of experimental data for the determination of ionization constants and solubility of the different type electrolytes are presented. Applications of the methods based on acid–base potentiometric titration and on determination of solubility–pH dependence of assayed substances are discussed. The original data on ionization constants and solubility of amoxicillin, cefprozil, cefatrizine, cephadroxil and initial β-lactams for production of cefaclor, cefprozil and cefatrizine, as well as solubility of by-product d-(−)-p-hydroxyphenylglycine are presented. Experimentally determined parameters and constants available in the literature for all abovementioned aminopenicillins and aminocephalosporins are collected. These data might be used for choice of the conditions of both processes: the enzymatic synthesis and the isolation of the product from reaction mixture.














Similar content being viewed by others
Notes
Correction of total titrant consumption on the volume that was spent specially for changing concentration of free H+ ions to adjust pH in the system is necessary under titration at pH values lower than 4.
Correction of total titrant consumption on the volume that was spent specially for changing concentration of free H+ ions to adjust pH in the system is necessary under titration at pH values higher than 10.
The characteristic solubility of an electrolyte is considered as solubility of its individual electro-neutral form that is zwitterionic form of amino acid or uncharged form of mono-carboxylic acid or monoamine.
References
Volpato G, Rodriges RC, Fernandez-Lafuente R (2010) Use of enzymes in production of semi-synthetic penicillins and cephalosporins: drawbacks and perspectives. Curr Med Chem 17:3855–3873
Kurochkina VB, Sklyarenko AV (2008) Enzymatic synthesis of beta-lactam antibiotics. Analytical review. In: Zaikov GE (ed) Biotechnology state of the art and prospects for development, Chapter 20. Nova Science Publishers, pp 175–204
Youshko MI, van Langen LM, de Vroom E, van Rantwijk F, Sheldon RA, Svedas VK (2001) Highly efficient synthesis of ampicillin in “aqueous solution-precipitate” system. Repetitive addition of substrates in semi-continuous process. Biotechnol Bioeng 73:426–430
Youshko MI, van Langen LM, de Vroom E, van Rantwijk F, Sheldon RA, Svedas VK (2002) Penicillin acylase-catalyzed ampicillin synthesis using a pH gradient: a new approach to optimization. Biotechnol Bioeng 78:589–593
Youshko MI, Moody HM, Bukhanov AL, Boosten WHJ, Svedas VK (2004) Penicillin acylase-catalyzed synthesis of β-lactam antibiotics in highly condensed aqueous systems: beneficial impact of kinetic substrate supersaturation. Biotechnol Bioeng 85:323–329
Ribeiro MPA, Fereira ALO, Giordano RLC, Giordano RC (2005) Selectivity of the enzymatic synthesis of ampicillin by E.coli PGA in the presence of high concentrations of substrates. J Mol Catal B Enzym 33:81–86
Basso A, Spizzo P, Toniutti M, Ebert C, Linda P, Gardossi L (2006) Kinetically controlled synthesis of ampicillin and cephalexin in highly condensed systems in the absence of a liquid aqueous phase. J Mol Catal B Enzym 39:105–111
Illanes A, Wilson L, Corrotea O, Tavernini L, Zamorano F, Aguirre C (2007) Synthesis of cephalexin with immobilized penicillin acylase at very high substrate concentrations in fully aqueous medium. J Mol Catal B Enzym 47:72–78
Illanes A, Wilson L, Aguirre C (2009) Synthesis of cephalexin in aqueous medium with carrier-bond and carrier-free penicillin acylase biocatalyst. Appl Biochem Biotechnol 157:98–110
Albert A, Serjeant EP (1971) The determination of dissociation constants. Chapman and Hall, London
de Levie R (2003) The Henderson–Hasselbalch equation: its history and limitations. J Chem Edu 80:146
Yamana T, Tsuji A (1976) Comparative stability of cephalosporins in aqueous solution: kinetics and mechanisms of degradation. J Pharmacol Sci 65:1563–1574
Tsuji A, Nakashima E, Yamana T (1979) Physicochemical properties of amphoteric β-lactam antibiotics II: solubility and dissolution behavior of aminocephalosporins as a function of pH. J Pharmacol Sci 68:308–311
Svedas VK, Margolin AL, Berezin IV (1980) Enzymatic synthesis of β-lactam antibiotics: a thermodynamic background. Enzym Microbiol Technol 2:138–144
Vieira MF, Barboza M, Giordano RLC (2003) Adsorption of components of enzymatic synthesis of ampicillin on different hydrophobic resins. Appl Biochem Biotechnol 105–108:705–713
Kurochkina VB, Nys PS (2003) Enzymatic synthesis of aminobeta-lactams. Physico-chemical bases of Down-Stream processes. Chem Pharm J (Russia) 37:30–37
Marino EL, Dominguez-Gil A (1981) Determination of the macro- and micro-ionization constants of a dipolar zwitterionic cephalosporin: cefadroxil. Int J Pharmacol 8:25–33
Moreno MN, Gonzalez JL, del Arco MA, Casado J (1990) Determination of macroscopic thermodynamic ionization constants under conditions of variable ionic strength by an optimization algorithm. Comput Chem 14:165–168
Nys PS, Elizarovskaya LM, Shellenberg NN, Savitskaya EM (1979) Solubility and complex forming properties of zwitterlites 6-aminocephalosporanic acid and 7-aminodesacetoxycephalosporanic acid. Antibiotics 24:895–902
Bulycheva MS, Nys PS, Savitskaya EM (1977) Physico-chemical properties of 7-aminoacetoxycephalosporanic acid. Antibiotics 22:1073–1076
Nys PS, Koltsova EV, Shellenberg NN (1976) Physico-chemical properties of 7-aminodesacetoxycephalosporanic acid. Antibiotics 21:391–394
Schroen CG, Nierstrasz VA, Kroon PJ et al (1999) Thermodynamically controlled synthesis of β-lactam antibiotics: equilibrium concentrations and side chain properties. Enzym Microbiol Technol 24:489–506
Kurochkina VB, Nys PS (2002) Kinetic and thermodynamic approach to design the processes for enzymatic synthesis of betalactams. Biocat Biotrans 20(1):35–41
Nys PS, Kurochkina VB (2000) Methodological approach to development of enzymatic technologies for semisynthetic betalactam antibiotic production. Appl Biochem Biotechnol A Enzym Eng Biotechnol 88:221–229
Dieter K, Ferhat I (2001) Synthesis of beta-lactam antibiotics with immobilized penicillin amidase. US Patent 6218138
Bruggink A (ed) (2001) Synthesis of β-lactam antibiotics: chemistry, biocatalysis and process integration. Kluver Academic Publishers, Dordrecht
Schroen CGPH, Nierstrasz VA, Bosma R et al (2002) Integrated reactor concepts for the enzymatic kinetic synthesis of cephalexin. Biotechnol Bioeng 80:144–155
Acknowledgments
The authors are grateful to Sichuan Industrial Institute of Antibiotics (China) for support of the work concerning study of the parameters for compounds involved in cefprozil and cefatrizine synthesis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurochkina, V.B., Sklyarenko, A.V., Satarova, J.E. et al. Ionization constants and solubility of compounds involved in enzymatic synthesis of aminopenicillins and aminocephalosporins. Bioprocess Biosyst Eng 34, 1103–1117 (2011). https://doi.org/10.1007/s00449-011-0560-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0560-9