Abstract
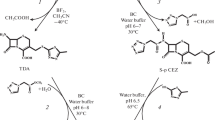
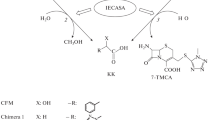
The efficiency has been compared of the optimized acylation processes of 7-aminocephalosporanic acid (7-ACA) and its С3 derivatives (7-amino-3-[2-methyl-1,3,4-thiadiazol-5-yl)-thiomethyl]-3-cephem-4-carboxylic acid (TDA), and 1-methyl-5-mercapto-1,2,3,4-tetrazolyl-7-aminocephalosporanic acid (7-TMCA)) by methyl esters of 1(H)-terazolylacetic and D-mandelic acids (METzAA and MEMA). These processes are catalyzed by immobilized cephalosporin-acid synthetase (IECASA) and are the biocatalytic stages of two different chemical-biocatalytic pathways for the synthesis of cefazolin (CEZ) and cefamandole (CFM). Biocatalytic acylation of 7-ACA resulting in the formation of semi-products of the synthesis of antibiotics proceeded more efficiently than acylation of the corresponding C3 derivatives of 7-ACA, leading to the formation of CEZ and CFM, in terms of both the achieved product yield and the possibility of obtaining compounds in high concentration in the reaction mixture. At the same time, the synthetase ability of IECASA is highest in the acylation of 7-ACA using METzAA. It was shown that the chemical-biocatalytic synthesis of CEZ and CFM by direct biocatalytic acylation of 7-ACA followed by chemical modification of the semi-product at the C3 position of the β-lactam nucleus is a promising alternative to the traditional pathway using the biocatalytic acylation of 7-ACA derivatives with a substituted 3-acetoxy group.






Similar content being viewed by others
REFERENCES
Pellis, A., Cantone, S., Ebert, C., and Gardossi, L., Evolving biocatalysis to meet bioeconomy challenges and opportunities, N. Biotechnol., 2018, vol. 40, pp. 154–169. https://doi.org/10.1016/j.nbt.2017.07.005
Rodriguez-Herrera, R., Puc, L.E.C., Sobrevilla, J.M.V., Luque, D., Cesar, S., Cardona-Felix, Aguilar-Gonzales, C.N., and Flores-Gallegos, A.C., Enzymes in the pharmaceutical industry for β-lactam antibiotic production, in Enzymes in Food Biotechnology—Production, Applications, and Future Prospects, Kuddus, M., Academic Press, 2018, vol. 36, pp. 627–643. https://doi.org/10.1016/B978-0-12-813280-7.00036-0
Cabri, W., Industrial synthesis design with low environmental impact in the pharma industry, in New Methodologies and Techniques for a Sustainable Organic Chemistry, Mordini, A. and Faigl, F., NATO Sci. Ser., 2008, vol. 246, pp. 119–129. https://doi.org/10.1007/978-1-4020-6793-8_6
Groger, H., Pieper, M., Koenig, B., Bayer, T., and Schleich, H., Industrial landmarks in the development of sustainable production processes for the β-lactam antibiotic key intermediate 7-aminocephalosporanic acid (7-ACA), Sustain. Chem. Pharm., 2017, vol. 5, pp. 72–79. https://doi.org/10.1016/j.scp.2016.08.001
Rudroff, F., Mihovilovic, M.D., Groger, H., Snajdrova, R., Iding, H., and Bornscheuer, U.T., Opportunities and challenges for combining chemo-and biocatalysis, Nat. Catal., 2018, vol. 1, pp. 12–22. https://doi.org/10.1038/s41929-017-0010-4
Sklyarenko, A.V., Eldarov, M.A., Kurochkina, V.B., and Yarotsky, S.V., Enzymatic synthesis of β-lactam acids (review), Appl. Biochem. Microbiol., 2015, vol. 51, no. 6, pp. 627–640. https://doi.org/10.1134/S0003683815060150
Kurochkina, V.B. and Sklyarenko, A.V., Enzymatic synthesis of beta-lactam antibiotics. Analytical review, in Biotechnology: State of the Art and Prospects for Development, Zaikov, G.E., Ed., Nova Science Publishers, 2008, vol. 20, pp. 175–204.
Kurochkina, V.B. and Sklyarenko, A.V., Enzymatic synthesis of beta-lactam antibiotics, Antibiot. Khimioter., 2005, vol. 50, nos. 5–6, pp. 39–58.
El'darov, M.A., Sklyarenko, A.V., Dumina, M.V., Medvedeva, N.V., Zhgun, A.A., Satarova, D.E., Sidorenko, A.I., Epremyan, A.S., and Yarotskii, S.V., Recombinant cephalosporin acid synthetase: optimization of expression in E. coli cells, immobilization and use for biocatalytic synthesis of cefazolin, Biomed. Khim., 2015, vol. 61, no. 5, pp. 646–651. https://doi.org/10.18097/PBMC20156105646
Wang, Lu., Sklyarenko, A.V., Duanhua, Li., Sidorenko, A.I., Zhao, C., Li, J., and Yarotsky, S.V., Enzymatic synthesis of cefazolin using immobilized recombinant cephalosporin acid synthetase as the biocatalyst, Bioprocess Biosyst. Eng., 2018, vol. 41, no. 12, pp. 1851–1867. https://doi.org/10.1007/s00449-018-2007-z
Deaguero, A.L., Blum, J.K., and Bommarius, A.S., Biocatalytic synthesis of β-lactam antibiotics, in Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology, Flickinger, M.C., Ed., Wiley, 2010, pp. 1–32. https://doi.org/10.1002/9780470054581.eib640
Sheldon, R.A. and Brady, D., The limits to biocatysis: pushing the envelope, ChemComm., 2018, vol. 54, pp. 6088–6104. https://doi.org/10.1039/c8cc02463d
Sklyarenko, A.V., Groshkova, I.A., Sidorenko, A.I., and Yarotskii, S.V., Alternative cefazolin synthesis with a cephalosporin-acid synthetase, Appl. Biochem. Microbiol., 2020, vol. 56, no. 5, pp. 526–537. https://doi.org/10.31857/S055510992005013X
Sklyarenko, A.V., Groshkova, I.A., Krest’yanova, I.N., and Yarotskii, S.V., Alternative synthesis of cefamandole with biocatalytic acylation catalyzed by immobilized cephalosporin-acid synthetase, Appl. Biochem. Microbiol., 2022, vol. 58, no. 3, pp. 251–260. https://doi.org/10.1134/S0003683822030127
Sklyarenko, A.V., Groshkova, I.A., Gorbunov, N.A., and Yarotskii, S.V., Biocatalytic synthesis of new cephalosporins using immobilized cephalosporin acid synthetase, Biotekhnologiya, 2022, vol. 38, no. 2, pp. 43–56.
Terreni, M., Ubiali, D., Pagani, G., Hernandez-Justiz, O., Fernandez-Lafuente, R., and Guisan, J.M., Penicillin G acylase catalyzed acylation of 7-ACA in aqueous two-phase systems using kinetically and thermodynamically controlled strategies: improved enzymatic synthesis of 7-[(1-hydroxy-1-phenyl)-acetamido]-3-acetoxymethyl-3-cephem-4-carboxylic acid, Enzyme Microb. Technol., 2005, vol. 36, nos. 5–6, pp. 672–679. https://doi.org/10.1016/j.enzmictec.2004.11.010
Fernandez-Lafuente, R., Guisan, J.M., Pregnolato, M., and Terreni, M., Immobilized enzymes and cells as practical catalysts, Tetrahedron Lett., 1997, vol. 38, no. 26, pp. 4693–4696.
Terreni, M., Pagani, G., Ubiali, D., Fernandez-Lafuente, R., Mateo, C., and Guisan, J.M., Modulation of penicillin acylase properties via immobilization techniques: one-pot chemoenzymatic synthesis of Cephamandole from Cephalosporin C, Bioorg. Med. Chem. Lett., 2001, vol. 11, no. 18, pp. 2429–2432. https://doi.org/10.1016/s0960-894x(01)00463-2
Terreni, M., Tchamkam, J., Sarnataro, U., Rocchietti, S., Fernandez-Lafuente, R., and Guisan, J.M., Influence of substrate structure on PGA-catalyzed acylations. Evaluation of different approaches for the enzymatic synthesis of cefonicid, Adv. Synth. Catal., 2005, vol. 347, no. 1, pp. 121–128. https://doi.org/10.1002/adsc.200404136
Fernandez-Lafuente, R., Rosell, C.M., and Guisan, J.M., The presence of methanol exerts a strong and complex modulation of the synthesis of different antibiotics by immobilized penicillin G acylase, Enzyme Microb. Technol., 1998, vol. 23, no. 5, pp. 305–310. https://doi.org/10.1016/S0141-0229(98)00053-2
Funding
The work was supported by an internal grant from the Kurchatov Institute National Research Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by I. Gordon
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: AA, acylating agent; 7-ACA, 7-aminocephalosporanic acid; CA, carboxylic acid; CASA, cephalosporin-acid synthetase; CEZ, Сefazolin; CFM, Сefamandole; EG, ethylene glycol; IECASA, immobilized cephalosporin-acid synthetase; KA, key amino acid; MA, D-mandelic acid; MEМА, D-mandelic acid methyl ester; METzAA, 1(H)-tetrazolylacetic acid methyl ester; PA, penicillin acylase; PB, phosphate buffer; TDA, (7-amino-3-[2-methyl-1,3,4-thiadiazol-5-yl)-thiomethyl]-3-cephem-4-carboxylic acid; 7-TMCA, 1-methyl-5-mercapto-1,2,3,4-tetrazolyl-7-aminocephalosporanic acid; TzAA, 1(H)-terazolylacetic acid.
Rights and permissions
About this article
Cite this article
Sklyarenko, A.V., Groshkova, I.A., Gorbunov, N.A. et al. A Comparative Study of Biocatalytic Acylation of 7-Aminocephalosporanic Acid and its C3 Derivatives. Appl Biochem Microbiol 59, 1089–1101 (2023). https://doi.org/10.1134/S0003683823080094
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823080094