Abstract
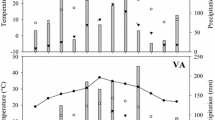
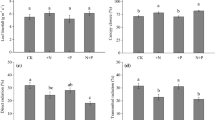
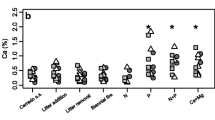
The theory of optimal leaf N distribution predicts that the C gain of plants is maximized when the N content per unit area (N area) scales with light availability, but most previous studies have demonstrated that the N distribution is not proportional to light availability. In tall trees, the leaves are often clustered on twigs (leaf cluster) and not evenly distributed within the crowns. Thus, we hypothesized that the suboptimal N distribution is partly caused by the limited capacity to translocate N between leaf clusters, and consequently, the relationship between light and N area differs for leaves in different clusters. We investigated the light availability and N content of all individual leaves within several leaf clusters on tall trees of a deciduous canopy species Fagus crenata in Japan. We observed that the within-cluster leaf N distribution patterns differed from the between-cluster patterns and the slopes of the relationships between light and N area were lower within clusters than between clusters. According to the detailed analysis of the N distribution within leaf clusters, N area was greater for current-year shoots with greater light availability or a larger total leaf area. The latter pattern was probably caused by the greater sink strength of the current-year shoots with a larger leaf area. These N distribution patterns suggest that leaf clusters are fairly independent with respect to their N use, and the productivity of real F. crenata crowns may be less than optimal.





Similar content being viewed by others
References
Anten NPR (2002) Evolutionarily stable leaf area production in plant populations. J Theor Biol 217:15–32
Anten NPR (2005) Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Ann Bot 95:495–506
Anten NPR, Schieving F, Medina E, Werger MJA, Schuffelen P (1995a) Optimal leaf area indices in C3 and C4 mono- and dicotyledonous species at low and high nitrogen availability. Physiol Plant 95:541–550
Anten NPR, Schieving F, Werger MJA (1995b) Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia 101:504–513
Anten NPR, Miyazawa K, Hikosaka K, Nagashima H, Hirose T (1998) Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plants in dense stands of a dicotyledonous herb. Oecologia 113:314–324
Barthelemy D, Caraglio Y (2007) Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Ann Bot 99:375–407
Bell AD (2008) Plant form: an illustrated guide to flowering plant morphology. Timber, Oregon
Buckley TN, Miller JM, Farquhar GD (2002) The mathematics of linked optimisation for water and nitrogen use in a canopy. Silva Fenn 36:639–669
Crawley MJ (2007) The R Book. Wiley, West Sussex
Dewar RC, Tarvainen L, Parker K, Wallin G, McMurtrie RE (2012) Why does leaf nitrogen decline within tree canopies less rapidly than light? An explanation from optimization subject to a lower bound on leaf mass per area. Tree Physiol 32:520–534
Ellsworth DS, Reich PB (1993) Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96:169–178
Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9–19
Falster DS, Westoby M (2005) Alternative height strategies among 45 dicot rain forest species from tropical Queensland, Australia. J Ecol 93:521–535
Falster DS, Warton DI, Wright IJ (2006) SMATR: standardised major axis tests & routines. http://www.bio.mq.edu.au/ecology/SMATR
Field C (1983) Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Fleck S, Niinemets Ü, Cescatti A, Tenhunen JD (2003) Three-dimensional lamina architecture alters light-harvesting efficiency in Fagus: a leaf-scale analysis. Tree Physiol 23:577–589
Hallé F, Oldeman RAA, Tomlinson PB (1978) Tropical trees and forests. Springer, Berlin Heidelberg New York
Hikosaka K (2004) Interspecific difference in the photosynthesis-nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hirose T, Werger MJA (1987a) Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72:520–526
Hirose T, Werger MJA (1987b) Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiol Plant 70:215–222
Hollinger DY (1996) Optimality and nitrogen allocation in a tree canopy. Tree Physiol 16:627–634
Iio A, Fukasawa H, Nose Y, Kato S, Kakubari Y (2005) Vertical, horizontal and azimuthal variations in leaf photosynthetic characteristics within a Fagus crenata crown in relation to light acclimation. Tree Physiol 25:525–536
Ishida A, Uemura A, Koike N, Matsumoto Y, Hoe AL (1999) Interactive effects of leaf age and self-shading on leaf structure, photosynthetic capacity and chlorophyll fluorescence in the rain forest tree, Dryobalanops aromatica. Tree Physiol 19:741–747
Kawamura K, Takeda H (2002) Light environment and crown architecture of two temperate Vaccinium species: inherent growth rules versus degree of plasticity in light response. Can J Bot 80:1063–1077
Kikuzawa K (1983) Leaf survival of woody plants in deciduous broad-leaves forests. 1. Tall trees. Can J Bot 61:2133–2139
Kikuzawa K (2003) Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Funct Ecol 17:29–38
Kimura K, Ishida A, Uemura A, Matsumoto Y, Terashima I (1998) Effects of current-year and previous-year PPFDs on shoot gross morphology and leaf properties in Fagus japonica. Tree Physiol 18:459–466
Kira T, Shinozaki K, Hozumi K (1969) Structure of forest canopies as related to their primary productivity. Plant Cell Physiol 10:129–142
Kull O (2002) Acclimation of photosynthesis in canopies: models and limitations. Oecologia 133:267–279
Kull O, Niinemets Ü (1993) Variations in leaf morphometry and nitrogen concentration in Betula pendula Roth., Corylus avellana L. and Lonicera xylosteum L. Tree Physiol 12:311–318
Kull O, Niinemets Ü (1998) Distribution of leaf photosynthetic properties in tree canopies: comparison of species with different shade tolerance. Funct Ecol 12:472–479
Le Roux X, Sinoquet H, Vandame M (1999) Spatial distribution of leaf dry weight per area and leaf nitrogen concentration in relation to local radiation regime within an isolated tree crown. Tree Physiol 19:181–188
Lloyd J, Patino S, Paiva RQ, Nardoto GB, Quesada CA, Santos AJB, Baker TR, Brand WA, Hilke I, Gielmann H, Raessler M, Luizao FJ, Martinelli LA, Mercado LM (2010) Optimisation of photosynthetic carbon gain and within-canopy gradients of associated foliar traits for Amazon forest trees. Biogeosciences 7:1833–1859
Meir P, Kruijt B, Broadmeadow M, Barbosa E, Kull O, Carswell F, Nobre A, Jarvis PG (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ 25:343–357
Milla R, Castro-Diez P, Maestro-Martinez M, Montserrat-Marti G (2005) Relationships between phenology and the remobilization of nitrogen, phosphorus and potassium in branches of eight Mediterranean evergreens. New Phytol 168:167–178
Millard P, Grelet G (2010) Nitrogen storage and remobilization by trees: ecophysiological relevance in a changing world. Tree Physiol 30:1083–1095
Niinemets Ü (1995) Distribution of foliar carbon and nitrogen across the canopy of Fagus sylvatica: adaptation to a vertical light gradient. Acta Oecol 16:525–541
Niinemets Ü (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30:1052–1071
Niinemets Ü (2012) Optimization of foliage photosynthetic capacity in tree canopies: towards identifying missing constraints. Tree Physiol 32:505–509
Niinemets Ü, Valladares F, Ceulemans R (2003) Leaf-level phenotypic variability and plasticity of invasive Rhododendron ponticum and non-invasive Ilex aquifolium co-occurring at two contrasting European sites. Plant Cell Environ 26:941–956
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Osada N (2006) Crown development in a pioneer tree, Rhus trichocarpa, in relation to the structure and growth of individual branches. New Phytol 172:667–678
Osada N (2013) Branching, nitrogen distribution, and carbon gain in solitary plants of an annual herb, Xanthium canadense. Plant Ecol 214:1493–1504
Osada N, Takeda H, Furukawa A, Awang M (2001) Leaf dynamics and maintenance of tree crowns in a Malaysian rain forest stand. J Ecol 89:774–782
Osada N, Tateno R, Mori A, Takeda H (2004) Changes in crown development patterns and current-year shoot structure with light environment and tree height in Fagus crenata (Fagaceae). Am J Bot 91:1981–1989
Parker GG (1995) Structure and microclimate of forest canopies. In: Lowman MD, Nadkarni NM (eds) Forest canopies. Academic Press, San Diego, pp 73–106
Peltoniemi MS, Duursma RA, Medlyn BE (2012) Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiol 32:510–519
Posada JM, Lechowicz MJ, Kitajima K (2009) Optimal photosynthetic use of light by tropical tree crowns achieved by adjustment of individual leaf angles and nitrogen content. Ann Bot 103:795–805
R Development Core Team (2011) R: a language and environment for statistical computing. Version 2.14.1. The R Foundation for Statistical Computing, Vienna
Sprugel DG, Hinckley TM, Schaap W (1991) The theory and practice of branch autonomy. Annu Rev Ecol Syst 22:309–334
Sterck FJ, Bongers F (2001) Crown development in tropical rain forest trees: patterns with tree height and light availability. J Ecol 89:1–13
Sumida A, Umeki K (2006) Stable foliage cluster (FC), a basic unit of the crown structure of tree species and its application to modeling of tree and forest structure: configuration of the FC model. In: International Sympodium on Plant Growth Modeling, Simulation, Visualization and Applications. IEEE Computer Society, pp 51–54
Suzuki AA (2003) Shoot growth patterns in saplings of Cleyera japonica in relation to light and architectural position. Tree Physiol 23:67–71
Thomas SC, Bazzaz FA (1999) Asymptotic height as a predictor of photosynthetic characteristics in Malaysian rain forest trees. Ecology 80:1607–1622
Traw MB, Ackerly DD (1995) Leaf position, light levels, and nitrogen allocation in five species of rain forest pioneer trees. Am J Bot 82:1137–1143
Uemura A, Ishida A, Nakano T, Terashima I, Tanabe H, Matsumoto Y (2000) Acclimation of leaf characteristics of Fagus species to previous-year and current-year solar irradiances. Tree Physiol 20:945–951
Uemura A, Ishida A, Tobias DJ, Koike N, Matsumoto Y (2004) Linkage between seasonal gas exchange and hydraulic acclimation in the top canopy leaves of Fagus trees in a mesic forest in Japan. Trees 18:452–459
Uemura A, Ishida A, Matsumoto Y (2005) Simulated seasonal changes in CO2 and H2O exchange at the top canopies of two Fagus trees in a winter-deciduous forest, Japan. For Ecol Manage 212:230–242
Uemura A, Harayama H, Koike N, Ishida A (2006) Coordination of crown structure, leaf plasticity and carbon gain within the crowns of three winter-deciduous mature trees. Tree Physiol 26:633–641
Van Pelt R, Sillett SC (2008) Crown development of coastal Pseudotsuga menziesii, including a conceptual model for tall conifers. Ecol Monogr 78:283–311
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev Camb Philos Soc 81:259–291
Watson MA, Casper BB (1984) Morphogenetic constraints on patterns of carbon distribution in plants. Annu Rev Ecol Syst 15:233–258
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas M-L, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Groom PK, Hikosaka K, Lee W, Lusk CH, Niinemets U, Oleksyn J, Osada N, Poorter H, Warton DI, Westoby M (2005) Modulation of leaf economic traits and trait relationships by climate. Global Ecol Biogeogr 14:411–421
Yamasaki M, Kikuzawa K (2003) Temporal and spatial variations in leaf herbivory within a canopy of Fagus crenata. Oecologia 137:226–232
Yamashita N, Koike N, Ishida A (2002) Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Environ 25:1341–1356
Yasumura Y, Hikosaka K, Matsui K, Hirose T (2002) Leaf-level nitrogen-use efficiency of canopy and understory species in a beech forest. Funct Ecol 16:826–834
Yasumura Y, Onoda Y, Hikosaka K, Hirose T (2005) Nitrogen resorption from leaves under different growth irradiance in three deciduous woody species. Plant Ecol 178:29–37
Yoda K (1978) Three-dimensional distribution of light intensity in a tropical rain forest of West Malaysia. Malay Nat J 30:161–177
Acknowledgments
We thank Kouki Hikosaka, Satoki Sakai, and Naoko Tokuchi for their valuable suggestions, and Yoko Osone, Ryuji Ichihashi, Yosuke Matsumoto, Soichiro Nagano, and Daisuke Sugiura for their help in field measurements. We also thank Ülo Niinemets and three anonymous referees for their helpful comments on the manuscript. This study was partly supported by a grant from the Ministry of Education, Science, Sports and Culture of Japan (18770011, 21780140 and 2437009), and the Nissan Foundation (08336).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ylo Niinemets.
Rights and permissions
About this article
Cite this article
Osada, N., Yasumura, Y. & Ishida, A. Leaf nitrogen distribution in relation to crown architecture in the tall canopy species, Fagus crenata . Oecologia 175, 1093–1106 (2014). https://doi.org/10.1007/s00442-014-2966-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-2966-y