Abstract
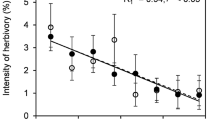
This study investigated spatio-temporal variation in the leaf area consumed by insect herbivores within a canopy of Fagus crenata, with reference to the light conditions of leaf clusters. There was no clear relationship between photosynthetic photon flux density (PPFD) and consumed leaf area (CLA) in May, immediately after leaf flush, but CLA decreased with an increase in PPFD after June. Leaf mass per area, carbon concentration, C/N ratio, concentration of total phenolics, and condensed tannin concentration were higher in leaves under high light intensity than those of leaves under low light. On the other hand, the nitrogen concentration of leaves decreased as light availability increased. Consequently, within-tree variation in light availability affects the consumption of leaves by insect herbivores through temporal changes in leaf characteristics.





Similar content being viewed by others
References
Agrell J, McDonald EP, Lindroth RL (2000) Effects of CO2 and light on tree phytochemistry and insect performance. Oikos 88:259–272
Ayres MP, Maclean SF Jr (1987) Development of birch leaves and the growth energetics of Epirrita autumnata (Geometridae). Ecology 68:558–568
Ayres MP, Clausen TP, MacLean SF Jr, Redman AM, Reichardt PB (1997) Diversity of structure and anti-herbivore activity in condensed tannins. Ecology 78:1696–1712
Bryant JP, Chapin FS III, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Choong MF (1996) What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Funct Ecol 10:668–674
Coley PD (1983) Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecol Monogr 53:209–233
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Damman H (1987) Leaf quality and enemy avoidance by the larvae of a pyralid moth. Ecology 68:88–97
Dudt JF, Shure DJ (1994) The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 75:86–98
Feeny P (1968) Effect of oak leaf tannins on larval growth of the winter moth Operophtera brumata. J Insect Physiol 14:805–817
Feeny P (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
Fortin M, Mauffette Y (2002) The suitability of leaves from different canopy layers for a generalist herbivore (Lepidoptera: Lasiocampidae) foraging on sugar maple. Can J For Res 32:379–389
Howard J (1990) Infidelity of leafcutting ants to host plants: Resource heterogeneity or defense induction? Oecologia 82:394–401
Hunter AF, Lechowicz MJ (1992) Foliage quality changes during canopy development of some northern hardwood trees. Oecologia 89:316–323
Kamata, N, Igarashi Y (1996) Seasonal and annual change of a folivorous insect guild in the Siebold's beech forests associated with outbreaks of the beech caterpillar, Quadricalcarifera punctatella (Motschulsky) (Lep., Notodontidae). J Appl Entomol 120:213–220
Larsson S, Ohmart CP (1988) Leaf age and larval performance of the leaf beetle Paropsis atomaria. Ecol Entomol 13:19–24
Larsson S, Wiren A, Lundgren L, Ericsson T (1986) Effects of light and nutrient stress on leaf phenolic chemistry in Salix dasyclados and susceptibility to Galerucella lineola (Coleoptera). Oikos 47:205–210
Lawrence RK, Mattson WJ, Haack RA (1997) White spruce and the spruce budworm: Defining the phenological window of susceptibility. Can Entomol 129:291–318
Lincoln DE, Mooney HA (1984) Herbivory on Diplacus aurantiacus shrubs in sun and shade. Oecologia 64:173–176
Lindroth RL, Reich PB, Tjoelker MG, Volin JC, Oleksyn J (1993) Light environment alters response to ozone stress in seedlings of Acer saccharum Marsh. and hybrid Populus L. III. Consequences for performance of gypsy moth. New Phytol 124:647–651
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Nabeshima E, Murakami M, Hiura T (2001) Effects of herbivory and light conditions on induced defense in Quercus crispula. J Plant Res 114:403–409
Nomura M, Itioka T (2002) Effects of synthesized tannin on the growth and survival of a generalist herbivorous insect, the common cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Appl Entomol Zool 37:285–289
Orians CM, Jones CG (2001) Plants as resource mosaics: a functional model for predicting patterns of within-plant resource heterogeneity to consumers based on vascular architecture and local environmental variability. Oikos 94:493–504
Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:223–230
Price ML, Butler LG (1977) Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J Agric Food Chem 25:1268–1273
Reich PB, Uhl C, Walters MB, Ellsworth DS (1991) Leaf lifespan as a determinant of leaf structure and function among 23 Amazonian tree species. Oecologia 86:16–24
Rhoades DF, Cates RG (1976) Toward a general theory of plant antiherbivore chemistry. In: Wallace JW, Mansell RL (eds) Biochemical interactions between plants and insects. Plenum, New York, pp 168–213
SAS (1985) SAS user's guide: statistics. Version 5 edn. SAS Institute, North Carolina
Schultz JC (1989) Tannin-insect interactions. In: Hemingway RW, Karchesy JJ (eds) Chemistry and significance of condensed tannins. Plenum, New York, pp 417–433
Thomas CD (1987) Behavioural determination of diet breadth in insect herbivores: the effect of leaf age on choice of host species by beetles feeding on Passiflora vines. Oikos 48:211–216
Wallin KF, Raffa KF (1998) Association of within-tree jack pine budworm feeding patterns with canopy level and within-needle variation of water, nutrient, and monoterpene concentrations. Can J For Res 28:228–233
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites (Methods in Ecology). Blackwell, Oxford
Williams KS (1983) The coevolution of Euphydryas chalcedona butterflies and their larval host plants. III. Oviposition behavior and host plant quality. Oecologia 56:336–340
Zucker WV (1982) How aphids choose leaves: the roles of phenolics in host selection by a galling aphid. Ecology 63:972–981
Acknowledgements
We would like to thank all staff members of the University Forest in Ashiu, Kyoto University, for enabling the investigation herein described. We are also grateful to all members of the Laboratory of Forest Biology, Kyoto University, Dr. Michinori Sakimoto of the Kyoto University Forest, and Dr. Shizuo Suzuki of the Institute for Environmental Sciences for their very valuable help, advice, and encouragement during the work. This work was supported by a Grant-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, of Japan (No. 11213202) and a Grant-in-aid for Scientific Research from the Japan Society for the Promotion of Science (No. 12304047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamasaki, M., Kikuzawa, K. Temporal and spatial variations in leaf herbivory within a canopy of Fagus crenata . Oecologia 137, 226–232 (2003). https://doi.org/10.1007/s00442-003-1337-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1337-x