Abstract
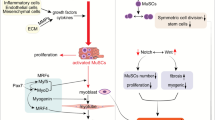
Fibrosis occurs in skeletal muscle under various pathophysiological conditions such as Duchenne muscular dystrophy (DMD), a devastating disease characterized by fiber degeneration that results in progressive loss of muscle mass, weakness and increased extracellular matrix (ECM) accumulation. Fibrosis is also observed after skeletal muscle denervation and repeated cycles of damage followed by regeneration. The ECM is synthesized largely by fibroblasts in the muscle connective tissue under normal conditions. Myofibroblasts, cells that express α-smooth muscle actin (α-SMA), play a role in many tissues affected by fibrosis. In skeletal muscle, fibro/adipogenic progenitors (FAPs) that express cell-surface platelet-derived growth factor receptor-α (PDGFR-α) and the transcription factor Tcf4 seem to be responsible for connective tissue synthesis and are good candidates for the origin of myofibroblasts. We show that cells positive for Tcf4 and PDGFR-α are expressed in skeletal muscle under normal conditions and are increased in various skeletal muscles of mdx mice, a murine model for DMD, wild type muscle after sciatic denervation and muscle subjected to chronic damage. These cells co-label with the myofibroblast marker α-SMA in dystrophic muscle but not in normal tissue. The Tcf4-positive cells lie near macrophages mainly concentrated in dystrophic necrotic-regenerating foci. The close proximity of Tcf4-positive cells to inflammatory cells and their previously described role in muscle regeneration might reflect an active interaction between these cell types and growth factors, possibly resulting in a muscular regenerative or fibrotic condition.






Similar content being viewed by others
Abbreviations
- α-SMA:
-
α-Smooth muscle actin
- Ang-1-7:
-
Angiotensin 1-7
- ECM:
-
Extracellular matrix
- DMD:
-
Duchenne muscular dystrophy
- FAPs:
-
Fibro/adipogenic progenitors
- PDGFR-α:
-
Platelet-derived growth factor receptor-α
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor type α
References
Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Munoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E (2014) Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-beta signalling. Hum Mol Genet 23:1237–1249
Andrade W, Brandan E (1991) Isolation and characterization of rat skeletal muscle proteoglycan decorin and comparison with the human fibroblast decorin. Comp Biochem Physiol B 100:565–570
Arnold L, Henry A, Poron F, Baba-Amer Y, Rooijen N van, Plonquet A, Gherardi RK, Chazaud B (2007) Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204:1057–1069
Batt J, Bain J, Goncalves J, Michalski B, Plant P, Fahnestock M, Woodgett J (2006) Differential gene expression profiling of short and long term denervated muscle. FASEB J 20:115–117
Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA (2013) Cellular dynamics in the muscle satellite cell niche. EMBO Rep 14:1062–1072
Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P, Cornelio F, Mantegazza R (1999) Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord 9:28–33
Brandan E, Gutierrez J (2013) Role of proteoglycans in the regulation of the skeletal muscle fibrotic response. FEBS J 280:4109–4117
Brandan E, Fuentes ME, Andrade W (1992) Decorin, a chondroitin/dermatan sulfate proteoglycan is under neural control in rat skeletal muscle. J Neurosci Res 32:51–59
Cabrera D, Gutierez J, Cabello-Verrugio C, Morales M, Mezzano S, Fadic R, Casar JC, Hancke JL, Brandan E (2014) Andrographolide attenuates skeletal muscle dystrophy in mdx mice and increases efficiency of cell therapy by reducing fibrosis. Skelet Muscle 4:6
Caceres S, Cuellar C, Casar JC, Garrido J, Schaefer L, Kresse H, Brandan E (2000) Synthesis of proteoglycans is augmented in dystrophic mdx mouse skeletal muscle. Eur J Cell Biol 79:173–181
Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E (2004) Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci 117:73–84
Ceco E, McNally EM (2013) Modifying muscular dystrophy through transforming growth factor-beta. FEBS J 280:4198–4209
Druker BJ (2004) Imatinib as a paradigm of targeted therapies. Adv Cancer Res 91:1–30
Fadic R, Brandan E, Inestrosa NC (1990) Motor nerve regulates muscle extracellular matrix proteoglycan expression. J Neurosci 10:3516–3523
Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E (2006) Increase in decorin and biglycan in Duchenne muscular dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med 10:758–769
Gargioli C, Coletta M, De Grandis F, Cannata SM, Cossu G (2008) PlGF-MMP-9-expressing cells restore microcirculation and efficacy of cell therapy in aged dystrophic muscle. Nat Med 14:973–978
Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA (2004) Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve 30:645–653
Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A (2013) Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153:376–388
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170:1807–1816
Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L (2009) Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J 23:2539–2548
Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12:153–163
Kumar A, Bhatnagar S, Kumar A (2010) Matrix metalloproteinase inhibitor batimastat alleviates pathology and improves skeletal muscle function in dystrophin-deficient mdx mice. Am J Pathol 177:248–260
Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM (2015) Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21:786–794
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P (2011) Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1:21
Marotta M, Sarria Y, Ruiz-Roig C, Munell F, Roig-Quilis M (2007) Laser microdissection-based expression analysis of key genes involved in muscle regeneration in mdx mice. Neuromuscul Disord 17:707–718
Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G (2011) Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138:371–384
Mezzano V, Cabrera D, Vial C, Brandan E (2007) Constitutively activated dystrophic muscle fibroblasts show a paradoxical response to TGF-beta and CTGF/CCN2. J Cell Commun Signal 1:205–217
Morales MG, Gutierrez J, Cabello-Verrugio C, Cabrera D, Lipson KE, Goldschmeding R, Brandan E (2013) Reducing CTGF/CCN2 slows down mdx muscle dystrophy and improves cell therapy. Hum Mol Genet 22:4938–4951
Moyer AL, Wagner KR (2011) Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol 23:568–573
Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G (2011) Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138:3625–3637
Olguin HC, Olwin BB (2004) Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: a potential mechanism for self-renewal. Dev Biol 275:375–388
Olson LE, Soriano P (2009)Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis.Dev Cell 16:303–313
Pannerec A, Formicola L, Besson V, Marazzi G, Sassoon DA (2013) Defining skeletal muscle resident progenitors and their cell fate potentials. Development 140:2879–2891
Pessina P, Cabrera D, Morales MG, Riquelme CA, Gutierrez J, Serrano AL, Brandan E, Munoz-Canoves P (2014) Novel and optimized strategies for inducing fibrosis in vivo: focus on Duchenne muscular dystrophy. Skelet Muscle 4:7
Porter JD, Merriam AP, Leahy P, Gong B, Khanna S (2003) Dissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin-deficient (mdx) mice. Hum Mol Genet 12:1813–1821
Porter JD, Merriam AP, Leahy P, Gong B, Feuerman J, Cheng G, Khanna S (2004) Temporal gene expression profiling of dystrophin-deficient (mdx) mouse diaphragm identifies conserved and muscle group-specific mechanisms in the pathogenesis of muscular dystrophy. Hum Mol Genet 13:257–269
Reed P, Bloch RJ (2005) Postnatal changes in sarcolemmal organization in the mdx mouse. Neuromuscul Disord 15:552–561
Riquelme C, Acuña MJ, Torrejon J, Rebolledo D, Cabrera D, Santos RA, Brandan E (2014) ACE2 is augmented in dystrophic skeletal muscle and plays a role in decreasing associated fibrosis. PLoS One 9:e93449
Robertson TA, Maley MA, Grounds MD, Papadimitriou JM (1993) The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 207:321–331
Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B (2013) Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31:384–396
Serrano AL, Mann CJ, Vidal B, Ardite E, Perdiguero E, Munoz-Canoves P (2011) Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol 96:167–201
Stedman H, Sarkar S (1988) Molecular genetics in basic myology: a rapidly evolving perspective. Muscle Nerve 11:668–682
Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298:R1173–R1187
Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12:143–152
Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S (2011) Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 124:3654–3664
Uezumi A, Ikemoto-Uezumi M, Tsuchida K (2014) Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Front Physiol 5:68
Whitehead NP, Yeung EW, Allen DG (2006) Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 33:657–662
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040
Zou Y, Zhang RZ, Sabatelli P, Chu ML, Bonnemann CG (2008) Muscle interstitial fibroblasts are the main source of collagen VI synthesis in skeletal muscle: implications for congenital muscular dystrophy types Ullrich and Bethlem. J Neuropathol Exp Neurol 67:144–154
Acknowledgments
The authors are grateful to Darling Vera, Lina Correa, Victor Troncoso and Ana Vasquez for their technical support and to Ximena Verges for help with the confocal microscopy.
Author contributions
O.C. conducted most of the experiments. O.C. and E.B. conceived the concepts, designed the study, analyzed the data and wrote the manuscript. D.R. and J.E.O. contributed to the denervation and chronic damage studies, respectively. H.O. helped with the Pax7 and NG2 cell type analyses. All authors read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
This study received financial support from the Fondecyt 1150106 grant to E.B., the CARE-PFB-12/2007 grant and Fondecyt 3140357 grant to D.R. and the Conicyt Doctoral Fellowship grant to O.C. and J.E.O.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOC 34 kb)
Table S2
(DOC 33 kb)
Fig. S1
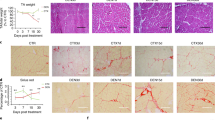
Increase in Tcf4-positive connective tissue fibroblasts in diaphragm of dystrophic skeletal muscle. a–i Representative confocal images showing a significant increase in interstitial Tcf4-positive cells (green) in cross-sections of the mdx muscle compared with the WT. Laminin-α2 (red) and nuclei (blue) are also stained (middle). e, f, k, l Representative confocal images of collagen type I immunostained in a cross-section of a WT and a mdx diaphragm. Nuclei (blue) are also stained. Bars 50 μm (top, middle), 100 μm (bottom). m, n Tcf4-positive cells were measured per field in the gastrocnemius and the tibialis anterior, respectively. Cells were counted at 60× magnification. Values are means ± SEM, n = 3 animals in each group. ***P < 0.001 WT vs mdx; with two-tailed Student’s t-test. o–r Western blot analysis of Tcf4, PDGFR-α and fibronectin in the gastrocnemius and tibialis anterior extracts obtained from the skeletal muscles of WT and mdx mice (5-months-old). The corresponding total Tcf4, PDGFR-α and fibronectin levels were measured by densitometry analysis in WT and mdx mice. Total GAPDH was used as a loading control. *P < 0.05, **P < 0.005, ***P < 0.0005 WT vs mdx; one-tailed Student’s t-test; n = 3 in each experimental group (GIF 107 kb)
Fig. S2
Architectural changes and fibrosis increase in skeletal muscles of adult mdx mice. Representative images of hematoxylin and eosin (top) and Sirius Red staining of total collagen (bottom) showing muscle architectural features in cryosections of the diaphragm, gastrocnemius and tibialis anterior muscles in WT and mdx skeletal muscle. Bars 50 μm (GIF 190 kb)
Fig. S3
Architectural changes and fibrosis increase in the gastrocnemius of adult WT mice following sciatic denervation. Cryosections of the gastrocnemius muscle in contralateral and denervated muscle, sampled 2 weeks after surgery. Representative images of hematoxylin and eosin staining (top), Sirius Red staining of total collagen (middle) and collagen type I immunofluorescence showing atrophic skeletal muscle architecture and increase in this fibrotic protein (bottom). Nuclei are stained with Hoechst (blue, bottom). Bars 50 μm (GIF 278 kb)
Fig. S4
Fibrosis induction in tibialis anterior muscle of adult WT mice following six repeated BaCl2 injections. Representative images of hematoxylin and eosin staining (top), Sirius Red staining of total collagen (middle), and collagen type I immunofluorescence (bottom) of WT tibialis anterior muscles subjected to six consecutive weekly rounds of BaCl2 injections (50 μl 1.2 % BaCl2), compared with six consecutive weekly rounds of saline injections (0.9 % NaCl), sampled 2 weeks after the final injection. Nuclei are stained with Hoechst (blue, bottom). Bars 100 μm (GIF 296 kb)
Fig. S5
Increase in NG2-positive pericytes in dystrophic and denervated skeletal muscles. a, b Representative confocal images showing that NG2-positive pericytes and interstitial Tcf4-positive cells are spatially located in different zones in WT diaphragm (serial cross-sections; dotted lines extracellular matrix [ECM]). Laminin-α2 (red) and nuclei (blue) are also stained. c, d Representative confocal images showing that NG2-positive pericytes and interstitial Tcf4-positive cells are spatially located in different zones in mdx tibialis anterior serial cross-sections. e–j Representative confocal images showing that NG2-positive pericytes increase in mdx when compared with WT muscles. These increases correlate with the degree of fibrosis found in the diaphragm, gastrocnemius and tibialis anterior muscles. k, l Representative confocal images showing that NG2-positive pericytes increase following denervation (2 weeks after sciatic denervation) when compared with those in contralateral gastrocnemius muscle. e–l Nuclei (blue) are also stained. Bars 50 μm (GIF 157 kb)
Rights and permissions
About this article
Cite this article
Contreras, O., Rebolledo, D.L., Oyarzún, J.E. et al. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res 364, 647–660 (2016). https://doi.org/10.1007/s00441-015-2343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2343-0