Abstract
Skeletal muscle is a highly specialized tissue composed of myofibres that performs crucial functions in movement and metabolism. In response to external stimuli and injuries, a range of stem/progenitor cells, with muscle stem cells or satellite cells (MuSCs) being the predominant cell type, are rapidly activated to repair and regenerate skeletal muscle within weeks. Under normal conditions, MuSCs remain in a quiescent state, but become proliferative and differentiate into new myofibres in response to injury. In addition to MuSCs, some interstitial progenitor cells (IPCs) such as fibro-adipogenic progenitors (FAPs), pericytes, interstitial stem cells expressing PW1 and negative for Pax7 (PICs), muscle side population cells (SPCs), CD133-positive cells and Twist2-positive cells have been identified as playing direct or indirect roles in regenerating muscle tissue. Here, we highlight the heterogeneity, molecular markers, and functional properties of these interstitial progenitor cells, and explore the role of muscle stem/progenitor cells in skeletal muscle homeostasis, aging, and muscle-related diseases. This review provides critical insights for future stem cell therapies aimed at treating muscle-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle, which comprises over 40% of the body's weight, performs crucial functions in vital activities such as locomotion, posture, metabolism and respiration [1,2,3,4]. It comprises non-dividing multinucleated myofibres and boasts an exceptional capacity for tissue regeneration, facilitated by MuSCs [5, 6]. These cells exhibit extraordinary adaptability and self-renewal capacities, as well as the ability to differentiate into various cells types, supporting muscle regeneration. In a quiescent state, the metabolic and transcriptional activity of MuSCs remain low, maintaining a G0 phase until external stimuli instigate their cell cycle entry towards the generation of committed progenitor cells. These cells can either differentiate into new myofibres for tissue repair or return to a quiescent state to replenish the MuSC pool [7,8,9]. Ageing, however, significantly diminishes the regenerative capacity of MuSCs due to disruptions in muscle tissue homeostasis, influenced by changes in the tissue microenvironment and intrinsic changes in the MuSCs, key factors modulating muscle tissue ageing [10, 11].
Numerous other muscle-derived interstitial progenitor cells (IPCs) have been identified encompassing fibro-adipogenic progenitors (FAPs), pericytes, PW1+/Pax7− interstitial progenitor cells (PICs), side population cells (SPCs), CD133 positive (CD133+) cells and Twist2 positive (TW2+ cells) cells [12, 13]. Muscle-specific mesenchymal stromal cells, FAPs, play a pivotal role in muscle regeneration, facilitating skeletal muscle repair post-injury [14,15,16]. Pericytes promote blood flow in conjunction with muscle cells, interacting with endothelial cells. Within skeletal muscle, pericytes embody mesenchymal progenitor cell characteristics, giving rise to adipocytes, chondrocytes, skeletal cells, muscle cells, and endothelial cells, contributing to the development of skeletal myofibres [17]. SPCs, another cohort of myogenic progenitor cells, are isolatable from mouse and human muscles, identified through their ability to exocytose Hoechst dye [18]. In the same vein, PICs expressing PW1 and lacking Pax7, demonstrate myogenic capacities, effectively contributing to skeletal muscle regeneration while simultaneously producing MuSCs and complementing PICs [19]. CD133+ cells emerge a type of multipotent stem cells derived from peripheral blood cells with myogenic potential [20]. Twist2+ cells contribute to the formation of specific fiber types during muscle homeostasis and regeneration [21]. These progenitor cells proliferate in response to muscle injury, with MuSCs, integral to skeletal muscle repair and regeneration, reliant on these muscle-resident interstitial progenitor cells. This review explores these stem/progenitor cells within skeletal muscle, examining their distinct functional properties, and delves into their roles within skeletal muscle homeostasis, ageing, and muscle-related diseases. An in-depth understanding of these cells’ function and regulation will assist in the development of effective muscle regeneration therapies.
MuSCs
Characteristics of MuSCs
Recent research indicates the presence of varying MuSC subpopulations, distinguishable by differing gene expression, cellular behavior and regenerative potential [22]. Single-cell sequencing experiments have unveiled clear disparities in the molecular and functional capabilities among these MuSC subpopulations [23, 24]. A large-scale single-cell sequencing study identified two separate clusters within satellite cells: one, a near-quiescent cluster characterised by the expression of Notch target Hes1, Gas1, Pax7, and Calcr, whilst the other cluster was enriched with Myf5, MyoD and ribosomal genes [25, 26]. These satellite cells possess remarkable myogenic potential, heavily relying on the expression of paired-homeobox transcription factors Pax3 and Pax7 followed by the expression of myogenic regulatory factors (MRFs) including MyoD and Myf5 [27]. Furthermore, MuSCs display notable functional variability, with some subpopulations demonstrating superior regenerative and self-renewal capabilities. Lineage tracing studies revealed that MuSCs subpopulations, which retained a label and expressed higher p27kip1 levels, were critical in maintaining a MuSCs bank and exhibited a strong regenerative capacity [28]. Single-cell RNA sequencing disclosed the existence of two MuSCs subpopulations during homeostasis and five myogenic cell subpopulations during the regeneration phase [29]. Intriguingly, one subpopulation showed enriched Notch2 receptor expression during both muscle homeostasis and regeneration. However, among three activated MuSCs subpopulations, one differentiating subpopulation was marked by suppressed Notch receptors expression and an overexpression of the Dll1 ligand, suggesting a potential role for a Dll1/Notch2 feedback loop in muscle growth regulation [29]. Consequently, through the application of cell sorting methods and single cell sequencing analysis, a wide range of MuSCs subpopulations has been uncovered, illuminating their considerable heterogeneity. This understanding paves the way for a more in-depth investigation of the cellular states within these subpopulations during muscle regeneration and their differing roles in this process.
MuSCs can be maintained through symmetric cell divisions and asymmetric cell divisions, which are important determinants of MuSCs fate. During symmetric division, two homogenous daughter cells evolve through a division parallel to the base, augmenting the number of MuSCs. Recently, Protein kinase C Theta (PKC θ) was found to can stimulate MuSCs to instigate symmetric division, regulating cell polarity during division and promoting self-renewal [30]. On the contrary, asymmetric division sees the Myf5− MuSCs undergo apical–basal division, a division perpendicular to the basal layer, producing a Myf5− stem cell at the apex and a Myf5+ progenitor cell at the basal position [31, 32]. Daughter stem cells inherit the original DNA from the mother cell, maintaining superior DNA information fidelity, while the newly synthesised DNA is allocated to daughter cells with higher differentiation potential [33]. As such, a satellite cell undergoing asymmetric division can generate a new satellite cell and a dedicated myogenic progenitor. Multiple studies have recently shown that epigenetic modifications during asymmetric division play an important role in programming the MuSCs towards myogenic commitment. Pax7 acetylation, which is regulated by the acetyltransferase MYST1 and the deacetylase SIRT2, governs the asymmetric division of MuSCs [34]. Coactivator-associated arginine methyltransferase 1 (Carm1) contributes to the self-renewal of MuSCs as well as muscle regeneration by methylating Pax7, facilitating Myf5 expression during asymmetric division of MuSCs [35]. Furthermore, epidermal growth factor/epidermal growth factor receptor (EGF/EGFR), mitogen-activated protein kinase (MAPK), and protease activating receptor (PAR) signalling pathways are essential for asymmetric division [36,37,38]. During regeneration, asymmetric segregation of embedded fate determinants prior to cell division can lead to varied daughter cell destinies. As a result, a carefully regulated equilibrium between self-renewal and differentiation is imperative to maintain the satellite stem cell pool and generate sufficient progenitors to support optimal muscle growth and regeneration.
Roles of MuSCs in skeletal muscle
Roles of MuSCs in skeletal muscle homeostasis and injury
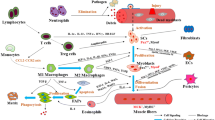
Under homeostatic conditions, MuSCs nestle quiescently between the basal lamina and the sarcolemma of myofibres, refraining from cell division. Trauma or pathological conditions within the muscle, however, trigger these cells into action, stimulating their activation and proliferation (Fig. 1). A subpopulation of these stimulated MuSCs is capable of differentiating into fusion-competent myoblasts that stimulate muscle regeneration [39, 40]. This activation process of MuSCs is propelled by the release of a spectrum of growth factors and cytokines, such as Hepatocyte Growth Factor (HGF), Fibroblast Growth Factor (FGF), Insulin-like Growth Factors (IGF-1/2), Epidermal Growth Factor (EGF), and Platelet-Derived Growth Factor-BB (PDGF-BB). These growth factors, secreted by inflammatory cells, endothelial cells, mesenchymal cells or stored bound to proteoglycans in the extracellular matrix (ECM), find release during ECM remodelling mediated by Matrix Metalloproteinases (MMPs) [41,42,43]. Having shifted from G0 to G1 phase, the activated MuSCs commence proliferation and differentiation into myogenic progenitor cells (MPCs). The resultant myoblasts conjoin, forming new myotubes that eventually mature into myofibres [44]. The myogenic differentiation of MuSCs is expertly steered by a family of sequence-specific transcription factors dubbed MRFs, comprising Myf5, MyoD, Myogenin and MRF4 (or MYF6). The transcription factor Pax7, characteristic of both quiescent and activated MuSCs also chips in, regulating the expression of MyoD and Myf5. Myf5, expressed in all quiescent MuSCs, subsequently instigates the expression of MyoD. This, in turn, goads the expression of myogenin and MRF4, vital agents for myogenic differentiation. Consequently, a subset of cells embarks on the myogenic programme hence becoming Pax7/MyoD-containing myogenic cells, which, while maintaining Pax7 positivity, downgrade MyoD, subsequently regenerating the necessary MuSCs for the subsequent round of muscle growth and regeneration [45]. These myogenic regulators, which play a central regulatory role during muscle development, get re-expressed during muscle regeneration to propel the myogenic differentiation of MuSCs.
Role of MuSCs in skeletal muscle injury and aging. Growth factors and cytokines released by inflammatory cells, endothelial cells and mesenchymal cells lead to the activation of MuSCs. MRFs then regulate the differentiation of MuSCs, leading to the formation of new myotubes by myoblasts, which eventually mature into myofibrils and cause muscle regeneration. During the ageing process, activation of the JAK-STAT and WNT signalling pathways and inhibition of Notch activity lead to a gradual decrease in the number of MuSCs and differentiation of MuSCs from myogenic to fibrogenic
MuSCs are pivotal in the growth and maintenance of skeletal muscle homeostasis. However, numerous studies reveal that adverse, pathological conditions within the muscle, including chronic injury and some severe myopathies, can dampen the activity, proliferation, and differentiation potential of MuSCs, thereby impacting muscle functionality [5, 46]. Chronic kidney disease has been found, for instance, to lessen the proliferation of MuSCs in muscles which is linked to a reduction in IGF-1 signalling and compromised differentiation [47, 48]. In parallel, muscle atrophy in Cachexia mice correlates to impaired MuSCs differentiation [49, 50]. Research unveiled increased apoptosis of MuSCs after activation in chronic obstructive pulmonary disease (COPD) patients’ muscles, and autophagy dysfunction in MuSCs is also seen in COPD [51, 52]. Our prior studies have indicated that inflammation and oxidative stress are significant factors in the advancement of muscle atrophy [53,54,55,56,57,58], we speculate that the shifts in MuSCs are closely tied to the inflammation and oxidative stress elicited by disease within the muscle microenvironment. Duchenne muscular dystrophy (DMD), caused by the absence of dystrophin encoded by the DMD gene, inflicts continuous degradation and regeneration on myofibres, driving inflammation, fibrosis and ultimately, deterioration of muscle mass and functionality [59, 60]. DMD patients' myofibres and MuSCs both express mutated dystrophin, creating anomalies in MuSC polarity, asymmetric division, and epigenetic regulation (Fig. 2) [61]. The cell polarity regulator Mark2 gets down-regulated in dystrophic MuSCs, resulting in the lack of Pard3 protein in the apical position, while Carm1 gets deactivated by p38γ resulting in a dive in Pax7 methylation and eventual suppression of Myf5 expression [35, 37]. Consequently, MuSCs lacking functional dystrophin exhibit aberrant asymmetric division and hindered myogenic differentiation [22]. One research found that reinstating dystrophin protects MuSCs from endoplasmic reticulum stress and oxidative stress, enhancing cell survival, proliferation, and differentiation [61]. Therefore, the deficiency of dystrophin goes beyond affecting differentiated myofibres; it can also restrict stem cell viability and functionality. Myasthenia gravis (MG) is an emerging autoimmune disease characterized by impaired neuromuscular transmission resulting in skeletal muscle contractile weakness. Myoblasts isolated from MG muscles, though initially more proliferative and differentiated than those from control muscles, eventually display a defective regenerative function [62]. These results imply that the autoimmune attacks in MG potentially impact myogenic signalling pathways, leading to adverse clinical outcomes. As such, enhancing the muscle microenvironment and by extension, optimising the function of MuSCs, could aid muscle regeneration. Implantation of exogenous stem cells also emerges as a promising therapeutic strategy for severe muscle diseases.
Roles of MuSCs in skeletal muscle aging
In the aging process, the number of MuSCs in muscles gradually decreases, resulting in a loss of their ability to self-renew and regenerate [63, 64]. This deficiency in muscle regeneration in aged individuals is largely attributed to an imbalance between symmetric and asymmetric splitting. Furthermore, MuSC differentiation into myotubes and myosin expression is reduced [65]. Consequently, when aging muscles sustain damage, the regenerative process is impaired, with limited proliferation of MuSCs and their progeny. This also results in delayed differentiation of progenitor cells into new myofibers and an increase in fibrosis. These factors collectively impact the efficiency of muscle regeneration [66]. Moreover, the reduced content of MuSCs may contribute to decreased capillary density in the skeletal muscle of aged organisms, further affecting muscle health [67]. Minor muscle damage caused by exercise has been found to increase the number of MuSCs in senescent muscle, thereby promoting their function and helping to maintain muscle health in older individuals [25]. Utilizing small molecule inhibitors such as nicotinamide N-methyltransferase inhibitors can enhance levels of NAD in aging skeletal muscle, consequently improving the proliferation and regeneration of aging MuSCs. This accelerated muscle regeneration can lead to enhanced skeletal muscle performance [68]. Therefore, modulation of MuSC function through exercise and medications holds potential as a strategy for treating age-related sarcopenia.
The pathways involved are characterised: JAK-STAT signalling, Notch signalling and Wnt signalling, all of which are associated with skeletal muscle ageing (Fig. 1). Previous research has highlighted the crucial role of the JAK-STAT signaling pathway in various muscle atrophy [56, 69,70,71]. In the context of aging muscles, an increased activity of the JAK-STAT signaling pathway hinders symmetric division, leading a decreased number of stem cells. Consequently, this impairs the regenerative capacity of the muscle [72]. The Notch signaling pathway, a major regulator in activating MuSCs and promoting proliferation, experiences a decline in activity with age. Dll1/Notch2 is vital for the self-renewal of MuSCs, and knockdown of Notch1 or Notch2 results in a reduced number of MuSCs in uninjured muscle, decreased proliferation, increased differentiation, and impaired muscle regeneration [29, 43]. Additionally, the Wnt signaling pathway plays a key role in inhibiting myogenic differentiation of senescent MuSCs. During aging, enhanced Wnt signaling activity alters the fate of satellite cell myogenic differentiation, diverting it towards fibrogenic differentiation [73, 74]. This shift leads to impaired muscle regeneration and increased fibrosis, further diminishing the muscle's repair capacity. Maintaining a balance between Notch and Wnt signaling is crucial. Initially, Wnt signaling is low when MuSCs are first activated, whereas the Notch signaling pathway is active. However, once a sufficient number of progenitor cells are generated, Wnt signaling increases in these cells, while Notch activity begins to decline [75]. Overactive Wnt signaling prompts the exit of MuSCs from the myogenic differentiation program and induces fibrosis [76]. These intercellular signaling pathways play a pivotal role in ensuring efficient skeletal muscle regeneration and maintaining homeostasis, both during aging and in the presence of diseases.
FAPs
Characteristics of FAPs
Located in the interstitial space of skeletal muscles, FAPs are mesenchymal stromal cells that play a pivotal role either in homeostatic conditions or during regeneration. These cells are chiefly identified by the positive expression of cell surface markers, such as PDGFRα/CD140α, Stem Cell Antigen-1 (Sca-1) and CD34, and lack the expression of Pax7 or any myogenic markers [77]. FAPs exhibit multipotent differentiation capacity—they can transform into adipogenic, fibrogenic, osteogenic and chondrogenic lineages [77, 78]. Furthermore, they also mediate immune responses and contribute to the remodelling of the extracellular matrix (ECM) [79, 80].
Interestingly, FAPs hold diverse differentiation potentials, enabling them to adopt various cell phenotypes and fates. Investigations utilizing scRNA-seq have disclosed the differentiation trajectory of FAPs, which is comparable to MuSCs. In intact muscles, FAPs cluster into two major groups—Dpp4+ FAPs and Cxcl14+ FAPs—with each further subdividing into specific cell population subsets [81]. The subsets exhibit distinct functional roles and responses to environmental cues in various muscle injury contexts [79, 82, 83]. Tie2+ PDGFα+ Sca-1+FAPs exhibited adipogenic and fibrogenic potential, which may be related to the infiltration of fat and fibrous tissue in chronic muscle diseases [84, 85]. For a mouse model of muscle injury, prompted by cardiotoxin-induction, activated FAPs display distinct transcriptional profiles at various time-points post-injury [83]. The muscles of mdx mice, wherein FAPs with high Sca1 expression dominate and exhibit greater adipogenic and proliferative capacities compared to those with lower Sca1 expression [82]. Strikingly, single-cell analysis of human skeletal muscle has identified discrete FAP subpopulations [86, 87]. For instance, in patients with Type 2 Diabetes (T2DM), a subpopulation of FAPs possessing CD90+ expression was found to be associated with the degenerative remodelling of the extracellular matrix [86]. Vcam1+ FAPs with fibrogenic potential were not only found to be instantly activated in acute injury accompanied by inflammatory response, but also found to be abnormally persistent in DMD muscles [85]. Another study has highlighted the heterogeneity of FAPs in patients with adipose infiltration, revealing that MME+ FAPs subpopulations exhibit high lipogenic potential. MME+ FAPs undergo apoptosis during muscle regeneration and differentiate into adipocytes under pathological conditions, leading to their depletion [87]. These novel insights into the roles of different FAP subpopulations during muscle injury and regeneration could ultimately contribute to our understanding of the molecular mechanisms underpinning these processes.
Roles of FAPs in skeletal muscle
Roles of FAPs in skeletal muscle homeostasis
Under homeostatic conditions, FAPs reside in a steady state within intact muscle, underpinning muscle growth and functionality. Recent work unraveled that this state of FAP quiescence is connected with the regulation by hypermethylated in cancer 1 (Hic1) [88, 89]. FAPs have been discovered to secrete extracellular matrix (ECM) constituents—collagen, laminin, and fibronectin—and thus contribute to the structure of the MuSC niche. Moreover, remodeling of the ECM, a process in which FAPs partake, is essential for the proliferative and self-renewal activities of MuSCs [90, 91]. In addition to their structural role, FAPs also generate cytokines and growth factors like growth differentiation factor 10 (GDF10, also known as bone morphogenetic protein 3b, Bmp3b) and interleukin-10 (IL-10), which are instrumental in myogenesis and muscle growth [92, 93].
Further illuminating the significance of FAPs, a study utilizing PDGFRαCreER knock-in mice demonstrated that a decrease in lean mass and grip strength became evident as early as 2-week post-FAP-depletion [94]. At 9-month post-FAP-depletion, there were notable reductions in the number of MuSCs, muscle weight, and the cross-sectional area (CSA) of individual myofibers [94]. This compelling evidence suggests that FAPs are indispensable for maintaining MuSC populations and normal muscle growth. Furthermore, FAPs appear to play a crucial role in preserving muscle fiber types and neuromuscular junctions. When FAPs are depleted, there is a rise in the ratio of slow-twitch fibers and defects in neural components [93]. Therefore, FAPs are critical to the preservation of muscle homeostasis.
Roles of FAPs in skeletal muscle injury
Upon muscle injury, FAPs become activated, taking on a crucial role in skeletal muscle injury and repair (Fig. 3). The changed muscle microenvironment disrupts the muscle niche homeostasis under the influence of external stimuli. FAPs transition out of their dormant state due to an increase in local inflammatory factors released by immune cells, leading to extensive proliferation. Meanwhile, FAPs can secrete inflammatory agents, which activate and recruit further immune cells, indirectly shaping the process of muscle regeneration. For instance, FAPs release interleukin 33 (IL-33), facilitating the recruitment of regulatory T-cells (Tregs) to the injury site [95, 96]. Simultaneously, FAPs can regulate the proliferation and differentiation of MuSCs through modulation of cytokine secretion, thus directly promoting muscle regeneration [94, 97]. Upon activation, FAPs can elevate the expression of WISP1 and conserve local MuSCs pools [98]. Moreover, FAPs engage in the phagocytosis of necrotic debris post-muscle injury, participate in ECM remodelling, and act as scaffolds guiding muscle neogenesis [99]. As such, FAP activation yields a positive tissue environment, synergistically promoting MuSC-mediated muscle regeneration.
Role of FAPs in skeletal muscle injury and aging. Upon muscle injury, FAPs become activated and have a dual role. On one hand, FAPs can regulate the proliferation and differentiation of MuSCs through modulation of cytokine secretion, thus directly promoting muscle regeneration. On the other hand, FAPs can also drive muscle fibrosis and adipose tissue accumulation if improperly regulated. Ageing pushes FAPs into a fibrotic state, while a decrease in levels of phosphorylated Akt leads to a decrease in their proliferative capacity; Furthermore, a reduction in WISP1 production in FAP cells leads to an impaired muscle-forming capacity in MuSCs, resulting in muscle atrophy
As the muscle regeneration progresses, FAPs are induced to undergo apoptosis by infiltrating macrophages through tumor necrosis factor (TNF) released via C–C chemokine receptor-2 (CCR2) [94]. This process serves to maintain the number of FAPs stable by eliminating their surplus. Insufficient clearance could lead to pathological accumulation of FAPs, which under pathological conditions including neuromuscular disorders and chronic disease, could precipitate profuse proliferation, differentiation, fat infiltration, and collagen deposition [100]. One experiment speculated that FAPs are not only associated with fibrosis and cytokine production during the early stages of muscle after glycerol injection, but are also involved in subsequent adipogenesis and fat accumulation [101]. Additionally, persistent FAP proliferation may result in their osteogenic differentiation into neurogenic heterotopic ossifications (NHOs), causing further muscle injury [102]. In denervated muscles, an accumulation of FAPs has been linked to persistent activation of the STAT3-IL6 signalling pathway, secreting high levels of IL6, inducing muscle atrophy and fibrosis [80]. In FAPs, PDGF-AA incites the activation of the RhoA pathway in FAPs isolated from DMD patients, potentiating proliferation, migration, and actin reorganization and encouraging the inception of the fibrotic process [103]. Additionally, autocrine/paracrine dysregulation of FAP adipogenesis can lead to fat infiltration (Fig. 2). Within FAPs, studies have shown that WNT5a inhibits peroxisome proliferator-activated receptor gamma (PPARγ) expression in a β-catenin-dependent manner, thereby orchestrating adipogenesis [104]. With an inhibition of the lipogenic and fibrogenic effects of FAPs, potential treatment opportunities for DMD arise. For instance, treatment studies conducted on mouse models of DMD using a complex kinase inhibitor blocking PDGFRα demonstrated muscle fibrosis reduction and improved muscle function [100, 105, 106]. Recent investigations have demonstrated that intramuscular administration of WNT7A can curtail adipogenesis in FAPs [107]. This is achieved by provoking the nuclear localization of Yes-associated protein (YAP) via Rho signaling in differentiated FAPs. Therefore, FAPs play a dual role in muscle diseases: while being supportive during muscle development and repair via interactions with MuSC and immune cells, excessive accumulation can result in undesirable outcomes, like intramuscular fat infiltration or fibrosis.
Roles of FAPs in skeletal muscle aging
Muscle aging is characterized by a decline in regenerative potential, often correlated with elevated levels of fibrosis and chronic inflammation [108,109,110]. Ageing pushes FAPs into a fibrotic state, while a decrease in levels of phosphorylated Akt leads to a decrease in their proliferative [79, 111]. Aged FAPs indirectly impact the myogenic potential of MuSCs following injury by restricting the secretion of myogenic factors, thereby diminishing the amplification and differentiation capacity of MuSCs [93, 98]. One study observed that during aging, abnormalities in extracellular matrix (ECM)-related factors secreted by FAP cells, specifically the diminished production of the matrix protein WISP1, resulted in impaired muscle-forming ability of MuSCs [98]. The addition of recombinant WISP1 protein to aged muscle improved muscle structure and fiber CSA, promoting the proliferation and differentiation of MuSCs and facilitating myogenesis. Furthermore, while the expression level of bone morphogenetic protein 3b (Bmp3b) significantly reduces during aging, administering recombinant Bmp3b in aged mice was shown to reverse their myasthenic phenotype [93]. A recent study discovered a deficiency in meteorin-like (Metrnl) protein, a type of myokine, in aged muscle following injury. Treating aged mice with recombinant Metrnl triggered FAP apoptosis via macrophage-derived TNF, strengthening the immune response to counteract the pro-fibrotic program, consequently improving regeneration of aging muscles [112]. In conclusion, FAPs play a vital role in aging skeletal muscle, and there is potential to restore the regenerative capacity of aging skeletal muscle in the future by targeting FAPs.
Pericytes
Characteristics of pericytes
Pericytes, perivascular pluripotent cells embedded within the capillary basal lamina, associate with capillary endothelial cells to stabilize capillary structure. They participate in the regulation of endothelial cell function and are instrumental in the formation of new capillaries and angiogenesis [113]. Given their regenerative capacity and tight association with endothelial cells, pericytes could prove therapeutically beneficial for diseases associated with vascular dystrophy, such as ischaemic stroke and muscular dystrophy [114].
Pericytes are identifiable by a multitude of molecular markers, including neural-glial antigen 2 (NG2), beta-type platelet-derived growth factor receptor (PDGFRβ), CD146, G protein signaling 5 (RGS5), nestin, α-SMA, desmin, CD13, and alkaline phosphatase (AP) [46, 115, 116]. However, employing multiple markers remains essential as these markers are not exclusive to pericytes, and their use is needed to purify pericyte populations from specific organs [117, 118]. Within skeletal muscles, pericytes are dividable into two primary subtypes based on the cellular marker expression: type I (PDGFRβ+NG2+CD146+ and Nestin−) and type II (PDGFRβ+NG2+CD146+ and Nestin+) [119]. Type I pericytes can differentiate into adipocytes and fibroblasts, which contributes to muscle function loss [120, 121]. Conversely, Type II pericytes possess myogenic potential and thus, contribute to skeletal muscle regeneration [122, 123]. One study found that laminin differentially orchestrates the proliferation and differentiation of type I and type II pericytes, inhibiting the proliferation of type II pericytes and exerting anti-lipogenic and myogenic impacts on type I and type II pericytes, respectively [124]. Additionally, pericytes isolated from adult skeletal muscle can in vitro differentiate into chondrocytes and osteocytes, contributing to skeletal muscle ossification [125, 126]. Consequently, different subtypes of pericytes play a heterogeneous role in skeletal muscle.
Roles of pericytes in skeletal muscle
Roles of pericytes in skeletal muscle homeostasis
Although the capillary network in adult tissues is largely stable, the capillaries in skeletal muscles undergo remodeling under physiological conditions to finely the supply of capillaries and to the dynamic metabolic demands imposed by skeletal cells. Pericytes, serving as players, contribute to both sprouting angiogenesis and non-sprouting angiogenesis, participating in the stability and remodeling of the system [127]. Moreover, pericytes also a crucial role within the microvascular niche satellite cells by promoting myogenesis and maintaining quiescence of stem cells through Ang1 secretion and IGF1-dependent activation [128]. Recent research has uncovered that when are subjected to a high-fat diet skeletal muscle PDGFRβ+ pericytes be stimulated to produce leptin thus enhancing the oxidation of fatty acids in muscle cells bolstering metabolic activity [129]. Considering the coordinating role of pericytes in the retina, experts speculate that pericytes also serve as coordinators within the capillary network and facilitate communication between capillaries and parenchymal cells [130]. Consequently, pericytes emerge pivotal actors in skeletal muscle homeostasis.
Roles of pericytes in skeletal muscle aging and injury
It has been demonstrated that young mice possess type 2 pericytes with inherent myogenic potential, while type 1 pericytes remain in a quiescent state [121]. Interestingly, as animals ageing, there is a notable decline in the myogenic capacity of type 2 pericytes, coinciding with an increased collagen production by type 1 pericytes. With aging, skeletal muscle capillaries thin, and perivascular coverage dwindles [131]. A recent study demonstrated a link between impaired brain capillary pericyte functionality and a decline in neurovascular performance in aging mice [131, 132]. Though research into pericytes' role in skeletal muscle aging is limited, these findings shed light on the dynamic behavior of pericyte subsets during the aging process and suggest a potential role for these cells in muscle regeneration and fibrotic remodeling.
In the event of skeletal muscle injury, muscle homeostasis is compromised, and pericytes undertake multifaceted roles across distinct microenvironments [118]. T2DM patients' muscles exhibit a disrupted connection between pericytes and endothelial cells, a condition associated with oxidative stress injury to the pericytes and mitochondrial dysfunction [133, 134]. Chronic limb ischemia, as observed in mouse models, may lead to pericyte disruption, as suggested by newly formed capillary structures showing abnormalities [135]. Therefore, abnormal pericyte can result in capillary dysfunction, possibly fuelling skeletal muscle injury. Multiple potential populations of myofibroblast progenitors have been associated with the progression of muscle fibrosis, among them FAPs as well as cells expressing specific markers such as a disintegrin and metalloprotease 1 (ADAM12) and glioma-associated oncogene 1 (Gli1). Notably, pericytes have been found to express these markers, thereby suggesting the possibility of certain subpopulations of pericytes contributing to the formation of myofibroblast progenitors [136, 137]. In patients suffering from peripheral artery disease, a thickening of the capillary basement membrane and pericapillary fibrosis in ischemic muscles could be ascribed to the differentiation of type I pericytes into fibroblasts [138]. Conversely, a study analysing pericyte changes in a mouse model of hind limb ischemia found ischemia triggered pericyte proliferation and migration and upregulated the expression of myogenic related transcripts [139]. Additionally, pericytes initiate expression changes in various cytokines, thus regulating the migration and activation of endothelial cells and inflammatory cells. It was reported that disuse had marginal effects on gene expression in muscle pericytes, but the number of NG2+Lin− pericytes decreased following immobilization [140]. A recent study found that CD146+Lin− pericytes display a higher capacity to restore type IIa fibre count after disuse compared to NG2+Lin− pericytes [141]. Intriguingly, CD146+ pericytes harbour the ability to secrete immune regulatory elements, drive angiogenesis and counteract fibrotic factors during skeletal muscle contraction, thereby actively reshaping the skeletal muscle [141, 142]. The subpopulation of pericytes has also been implicated in the accumulation and infiltration of fat in diseased skeletal muscle, including conditions such as obesity, dystrophies, and aging (Fig. 2). Recent research finding indicate that type-1 pericytes undergo adipogenesis, thereby actively participating in the deposition of intramuscular adipose tissue (IMAT) and the subsequent fatty degeneration of skeletal muscle [124]. In a model of congenital muscular dystrophy, PDGFRβ+ pericytes differentiate into perilipin+ adipocytes in skeletal muscle in vivo, and laminin enhances PDGFRβ+ pericytes myogenesis while inhibiting adipogenesis via gpihbp1 [143]. Meanwhile, in dystrophic muscles, AP+ pericytes, at the preliminary stage of muscle degeneration or regeneration, temporarily expand and synergise their efforts with MuSCs and other muscle stem cells to sustain a continuous demand for new myofibres. Nevertheless, over time, the number of AP+ pericytes dwindles and their myogenic capacity significantly weakens (Fig. 2) [144, 145].
Currently, pericytes and pericyte-like cells under the spotlight in trials pertinent to the treatment of DMD [146, 147]. Pericytes’ transplantation has also been found to recover the myofibre CSA following immobilization/remobilization, suggesting the potential pericytes’ transplantation to enhance disuse skeletal muscle recovery [140]. In addition, one study leveraged human embryonic stem cells to generate PDGFRβ+PDGFRα– subtypes of non-fibro-adipogenic, non-myoblastic and pericyte-like derivatives. These surrogate cells, post-transplantation, reclaimed the myogenic matrix niche—a hopeful step towards cell therapy that could address chronic muscle diseases [148]. Emerging evidence suggests pericytes in disused muscles in mice are dysfunctional and lack antioxidant defenses. Treating healthy pericytes with hydrogen peroxide prompts the release of protein vesicles teeming with anti-inflammatory and antioxidant activities, causing a recovery of skeletal muscle fibre size and remodeling of the extracellular matrix in young adult and elderly mice affected by muscle disuse [149].
In summary, compelling evidence has emerged implicating pericytes in their ability to differentiate into myogenic cells and facilitate muscle repair following injury. Conversely, it has also been observed that pericytes are capable of differentiating into adipocytes and fibroblasts, thereby contributing to muscle degeneration. These intricate and dual roles of pericytes in both regenerative and degenerative processes underscore the complexity of their involvement in muscle homeostasis and pathology. As such, gaining a more nuanced understanding of pericytes' subdivisions and their evolutionary potential is critical for architecting targeted pericyte therapies and treating conditions associated with muscle injury.
PICs
Characteristics of PICs
PICs constitute a subset of muscle-resident stem cells that express the cellular stress mediator protein PW1. Given that satellite cells also express PW1, the location and molecular markers serve to differentiate PICs from satellite cells. Satellite cells express Pax7 and are located under the basal layer, while PICs do not express Pax7 and are positioned within in the interstitium [12]. Operating as muscle mesenchymal cells, PICs can also express the muscle-specific progenitor cell marker CD34, but not endothelial markers like CD31 [150].
A study systematically analyzed the characteristics of PICs and found that they can proliferate in vitro for a long time while maintaining a stable phenotype [12]. They are clonogenic, have self-renewal capabilities, and are non-tumorigenic. PICs possess the remarkable capacity to break through germ layer boundaries, contributing to the generation of cell types hailing from all three lineages, both in vitro and in vivo. Despite their versatility, PICs exhibit a distinct affinity towards the mesoderm lineage, overshadowing the endodermal or ectodermal ones, in an in vivo context. As bi-potent cells, PICs can differentiate into smooth muscle cells and skeletal myofibres, which are cellular entities typically originating from the mesoderm layer. Notably, clonal PIC cell lines primarily differentiate into skeletal muscles. A previous study also demonstrated the myogenic potential of SCA1+PICs, with PICs expressing moderate levels of SCA1 emerging within 3 weeks of birth, while those expressing high levels of SCA1 crop up post-birth and during adulthood [151]. Adult PICs can be categorised into two distinct groups: the majority, expressing PDGFRα, possess the potential for adipogenic/fibrogenic differentiation, while a smaller subset demonstrates myogenic potential. Echoing the pattern observed in pericytes, laminin has also been identified as a regulator of PDGFRβ + PIC differentiation via gpihbp1 [143]. Therefore, distinct types of PICs exhibit diverse functions in skeletal muscle.
Roles of PICs in skeletal muscle homeostasis, aging and injury
PICs serve a pivotal role in maintaining postnatal muscle growth and muscle homeostasis [151]. They also perform duties as secretory cells, dispensing a range of growth factors that fuel muscle repair. The administration of allogeneic porcine PICs into damaged skeletal muscle enhances and hastens the regeneration of myofibres and neocapillarization [152]. This repair effect hinges on the stimulation of the endogenous stem cell pool and the promotion of autologous skeletal muscle repair and regeneration. The reparative impact of PICs can be partially attributed to the cytokines they secrete, including CCL2, TIMP-1, TIMP-2 and so on (Table 1). Intriguingly, the injection of Sca-1+ PICs, derived from mouse skeletal muscle, into the myocardial marginal zone can curtail myocardial remodelling post-myocardial infarction [153]. This protective effect is correlated with PICs expressing and secreting growth factors such as VEGFA, TGF-β, FST, IGF-1, and HGF. Therefore, PICs potentiate muscle regeneration by secreting growth factors that enhance the microenvironment conducive to muscle regeneration.
Additionally, a study has found a decrease in the number of PICs in muscles with ageing [12]. It is noteworthy that the high expression of growth differentiation factor-11 (GDF-11) in PICs has been shown to improve aging-related dysfunction in skeletal muscle by restoring the function of muscle stem cells, suggesting that PICs may also play a role in skeletal muscle aging [152, 154]. In summary, PICs can not only expand and differentiate into myoblasts in vitro, but also effectively promote skeletal muscle regeneration in vivo, making them the potential stem cells for the treatment of muscle atrophy.
Other interstitial progenitor cell populations
SPCs
SPCs are a kind of muscle-derived stem cells, which expressed Sca-1, a hematopoietic stem cell marker [155]. Promptly found across various adult mammalian tissues and organs, these cells actively participate in the rejuvenation of locally damaged tissues witnessed across numerous tissues [155, 156]. Muscle SPCs that reside within the perivascular stromal realm of skeletal muscles demonstrating pronounced heterogeneity [157, 158]. Notably, skeletal muscle-derived SPCs comprise three distinct subgroups: CD31+CD45− SPCs, CD31−CD45+ SPCs, and CD31−CD45+SPCs [159]. CD31+CD45− SPCs account for the vast majority of SPCs in untreated skeletal muscles, exhibiting the phenotype of endothelial cells. Nonetheless, their ability to proliferate and differentiate after injury or in vitro culture exhibits a lesser extent [159, 160]. CD31−CD45+ SPCs, endowed with myogenic potential, have been implicated in the formation of muscle fibers. CD31−CD45− SPCs display traits similar to mesenchymal stem cells in undamaged skeletal muscles, but amplifies their proliferation promptly, showing maximum myogenic potential, following muscle injury [159]. Not only that, but these cells also show an upregulated expression of regeneration regulatory factors to amplify muscle regeneration. Upon injection of myoblasts with CD31−CD45− SPCs into the anterior tibialis muscle of mdx mice, CD31−CD45− SPCs demonstrated a bolstered ability to stimulate the proliferation and migration of myoblasts in vivo [161]. On the other hand, CD31−CD45− SPCs also have a high tendency to differentiate into osteoblasts or adipocytes in vitro, suggesting that they may contribute to muscle degeneration in some muscle diseases such as DMD. Additionally, following muscle injury, the number of ABGC2+SPC increased, predominantly differentiating into endothelial cells and participating in muscle regeneration [162]. However, one study pointed the SP cells extracted from dystrophic or cardiotoxin-injured muscle, unable to undergo myogenesis, but instead differentiate into FAP cells [163]. This indicates that further investigation into the specific roles of different SPC subtypes in muscle injury and regeneration seems crucial.
CD133+ cells
CD133+ cells represent an additional variety of pluripotent stem cells, having their origins traced back to peripheral blood [20]. Research has indicated that muscular CD133+ cells comprise progenitor cells of myoblast, myoendothelial cells, pericytes, and fibroblasts [164]. Notably, some Human CD133+ (hCD133+) cells have been discovered beneath the basal layer of muscle fibers, expressing myogenic markers [165, 166]. The hCD133+ cells, when cultured in vitro, have demonstrated the capacity to form myotubes, and when transplanted into immune-deficient mice, they have shown the potential to form functional muscle stem cells [165]. A compelling finding has been noted in a mouse cryoinjury model wherein CD133+ cells have outperformed myoblasts in their migratory, proliferative, and overall regenerative capabilities [167]. Clinical trials exploring autologous CD133+ cell transplantation in patients with DMD have led to increased muscle vascularisation and transition of myosin-positive muscle fibers from slow to fast, but no notable improvements in muscle function [168]. The recent study showcased a comparative analysis of CD133+ cells derived from DMD and normal human muscles, and their ability to promote muscle regeneration [164]. DMD CD133+ cells were found to have a reduced potential to undergo myogenic differentiation in vitro compared to the CD133+ cells derived from normal muscles. Furthermore, post intramuscular transplantation into immune-deficient mouse muscles, satellite cells were not generated, and a significantly fewer number of donor-derived muscle fibers were produced. Hence, the need for comprehensive investigation into the functions of various subgroups of CD133+ cells becomes apparent to leverage the therapeutic potential of CD133+ cells in treating muscle-related disease.
Tw2+ cells
The transcription factor Twist is expressed in muscle progenitor cells during embryogenesis, playing an integral role in mesoderm development and muscle formation [169]. Twist1 and Twist2, in vertebrates, are detected in an array of mesenchymal cellular types, not in differentiated myofibers. Mesenchymal cells expressing Twist2 represent a group of myogenic progenitor cells that contribute to specific fiber types during muscle homeostasis and regeneration, and contribute to the formation of type IIb/x myofibers in adult muscles [21]. However, Tw2+ cells did not contribute to the primary or secondary myogenesis in embryos. The ablation of Tw2+ cells leads to specific atrophy of IIb type fibers, supporting the view that Tw2+ cells are important for maintaining IIb type myofibers size in adulthood [170, 171]. Tw2+ cells fuse specifically with fast-twitch myofibers, mainly due to the chemorepulsion system mediated by Sema3a and Nrp1 signaling. Nrp1 is a cell surface marker of Tw2+ cells, and Sema3a is a chemorepellent ligand for Nrp1, is only expressed by type I and IIa myofibers, but not by type IIb myofibers [172]. The study also found that Tw2+ cells can autonomously initiate muscle generation during the regeneration process and can fuse with themselves and MuSCs [170]. Tw2+ progenitor cells are similar in molecular and anatomical aspects to FAPs, but differ from Pax7+ MuSCs. Pax7+ MuSCs located beneath the basal lamina of the myofibres, contributing to all myofibers types, while Tw2+ progenitor cells reside in the muscle interstitium, with contributions solely to type IIb/x myofibers [170, 173]. PDGFRα and PDGFRβ are highly enriched in Tw2+ cells, but almost not detected in Pax7+ cells, indicating that Tw2+ cells are different from Pax7+ MuSCs. However, Tw2+ cells removed from their native environment and cultured in vitro can obtain the fate of Pax7+ cells and exhibit myogenic potential similar to Pax7+ cells [170]. Examination of skeletal muscle biopsy samples from young and elderly individuals showed that Tw2+ cells were more common in elderly muscles and increased after 12 weeks of resistance exercise training in humans. However, the number of Tw2+ cells is not related to muscle mass or myofiber CSA size, and their abundance is negatively correlated with CSA and myonuclear domain size after resistance exercise training [174]. This study shows that although Tw2+ cells respond to aging and exercise, their myogenic potential still needs further research. In summary, myogenic progenitor cells expressing Tw2 represent a previously unrecognized, fiber-type specific stem cell that participates in postnatal muscle growth and regeneration. Nevertheless, an in-depth exploration of the role of Tw2+ cells in muscle injuries, aging, and various muscle diseases remains to be unveiled.
Conclusions and perspectives
Skeletal muscle stem cells/progenitor cells play their respective roles whether it is in a stable state or subjected to varying degrees of damage (Table 2). The heterogeneity and division mode of MuSCs affect their regulatory role in skeletal muscle. When muscles experience stimulations and trauma, these cells activated and proliferated, with a subset differentiate into myoblasts, thus aiding in skeletal muscle repair and regeneration. With PICs portraying attributes such as robust clonality, self-renewal, pluripotency, non-tumorigenicity, and the capacity to secrete abundant muscle-regenerative factors, they arise as ideal choices for transplantation cells aimed at muscle injury recovery. The dual-differentiation feature of FAPs, when judiciously regulated, aids in the repair of skeletal muscle damage. Nevertheless, an excessive proliferation of FAPs can deliver more damaging consequences to aging skeletal muscles. Hence, monitoring the dynamic interplay between cellular entities, cytokines, and growth factors is indispensable in ensuring undisturbed stem cell function. Although the proportion of pericytes, SPCs, and CD133+ cells in skeletal muscle is relatively small, some of their subpopulations exhibit myogenic potential and secrete muscle regeneration nutrient factors, thereby collaborating with other cells to maintain skeletal muscle homeostasis and participate in muscle regeneration. Additionally, the recently recognized Tw2+ cells, contributors to specific fiber types during muscle homeostasis and regeneration, warrant further investigative light into their distinct regulatory mechanisms. Muscle atrophy including DMD, being the most prevalent skeletal muscle diseases, pose significant harm, while the damaging effects of skeletal muscle aging on human health cannot be overlooked. In the context of muscle atrophy, there is an intricate and dynamic dialogue, that transpires among various cell types within the muscular microenvironment. Each cellular participant in this dialog plays a role in the complex narrative of muscle decline and potential recovery. Crosstalk in muscle atrophy among MuSCs, pericytes, FAPs, PICs, SPCs, and CD133+ cells is altered, potentially leading to a breakdown in the normal regenerative processes and a shift towards muscle wasting. A more in-depth exploration of the biological characteristics of different muscle stem cell subpopulations, as well their functional mechanisms in the skeletal muscle ecosystem—especially the inter-cellular coordination mechanism—can emerge as a promising investigation, thereby developing more targeted cell therapy interventions to improve skeletal muscle function in diseases related to this muscular system.
Data availability
Not applicable.
References
Huang L, Li M, Deng C, Qiu J, Wang K, Chang M, Zhou S, Gu Y, Shen Y, Wang W et al (2022) Potential therapeutic strategies for skeletal muscle atrophy. Antioxidants (Basel, Switzerland) 12(1):44
Chen X, Ji Y, Liu R, Zhu X, Wang K, Yang X, Liu B, Gao Z, Huang Y, Shen Y et al (2023) Mitochondrial dysfunction: roles in skeletal muscle atrophy. J Transl Med 21(1):503
Zhang H, Qi G, Wang K, Yang J, Shen Y, Yang X, Chen X, Yao X, Gu X, Qi L et al (2023) Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol 214:115664
Sun J, Zhou H, Chen Z, Zhang H, Cao Y, Yao X, Chen X, Liu B, Gao Z, Shen Y et al (2023) Altered m6A RNA methylation governs denervation-induced muscle atrophy by regulating ubiquitin proteasome pathway. J Transl Med 21(1):845
Wong A, Pomerantz JH (2019) The role of muscle stem cells in regeneration and recovery after denervation: a review. Plast Reconstr Surg 143(3):779–788
de Morree A, Rando TA (2023) Regulation of adult stem cell quiescence and its functions in the maintenance of tissue integrity. Nat Rev Mol Cell Biol 24(5):334–354
Garcia-Prat L, Sousa-Victor P, Munoz-Canoves P (2017) Proteostatic and metabolic control of stemness. Cell Stem Cell 20(5):593–608
Fuchs E, Blau HM (2020) Tissue stem cells: architects of their niches. Cell Stem Cell 27(4):532–556
Relaix F, Bencze M, Borok MJ, Der Vartanian A, Gattazzo F, Mademtzoglou D, Perez-Diaz S, Prola A, Reyes-Fernandez PC, Rotini A et al (2021) Perspectives on skeletal muscle stem cells. Nat Commun 12(1):692
Dumont NA, Wang YX, Rudnicki MA (2015) Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142(9):1572–1581
Blau HM, Cosgrove BD, Ho AT (2015) The central role of muscle stem cells in regenerative failure with aging. Nat Med 21(8):854–862
Cottle BJ, Lewis FC, Shone V, Ellison-Hughes GM (2017) Skeletal muscle-derived interstitial progenitor cells (PICs) display stem cell properties, being clonogenic, self-renewing, and multi-potent in vitro and in vivo. Stem Cell Res Ther 8(1):158
Dunn A, Talovic M, Patel K, Patel A, Marcinczyk M, Garg K (2019) Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res 37(6):1246–1262
Sastourne-Arrey Q, Mathieu M, Contreras X, Monferran S, Bourlier V, Gil-Ortega M, Murphy E, Laurens C, Varin A, Guissard C et al (2023) Adipose tissue is a source of regenerative cells that augment the repair of skeletal muscle after injury. Nat Commun 14(1):80
Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12(2):153–163
Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12(2):143–152
Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21(2):193–215
Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC (1999) Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401(6751):390–394
Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA (2010) Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol 12(3):257–266
Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K et al (2004) Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Investig 114(2):182–195
Cameron A, Wakelin G, Gaulton N, Young LV, Wotherspoon S, Hodson N, Lees MJ, Moore DR, Johnston AP (2022) Identification of underexplored mesenchymal and vascular-related cell populations in human skeletal muscle. Am J Physiol Cell Physiol 323(6):C1586-c1600
Feige P, Brun CE, Ritso M, Rudnicki MA (2018) Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell 23(5):653–664
Andre AB, Rees KP, O’Connor S, Severson GW, Newbern JM, Wilson-Rawls J, Plaisier CL, Rawls A (2023) Single cell analysis reveals satellite cell heterogeneity for proinflammatory chemokine expression. Front Cell Dev Biol 11:1084068
Barruet E, Garcia SM, Striedinger K, Wu J, Lee S, Byrnes L, Wong A, Xuefeng S, Tamaki S, Brack AS et al (2020) Functionally heterogeneous human satellite cells identified by single cell RNA sequencing. Elife 9:e51576
Sousa-Victor P, Garcia-Prat L, Munoz-Canoves P (2022) Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat Rev Mol Cell Biol 23(3):204–226
Dell’Orso S, Juan AH, Ko KD, Naz F, Perovanovic J, Gutierrez-Cruz G, Feng X, Sartorelli V (2019) Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development 146(12):dev174177
Weintraub H, Davis R, Tapscott S, Thayer M, Krause M, Benezra R, Blackwell TK, Turner D, Rupp R, Hollenberg S et al (1991) The myoD gene family: nodal point during specification of the muscle cell lineage. Science 251(4995):761–766
Chakkalakal JV, Christensen J, Xiang W, Tierney MT, Boscolo FS, Sacco A, Brack AS (2014) Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development 141(8):1649–1659
Yartseva V, Goldstein LD, Rodman J, Kates L, Chen MZ, Chen YJ, Foreman O, Siebel CW, Modrusan Z, Peterson AS et al (2020) Heterogeneity of satellite cells implicates DELTA1/NOTCH2 signaling in self-renewal. Cell Rep 30(5):1491-1503.e1496
Benedetti A, Fiore PF, Madaro L, Lozanoska-Ochser B, Bouche M (2020) Targeting PKCtheta promotes satellite cell self-renewal. Int J Mol Sci 21(7):2419
Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129(5):999–1010
Wang YX, Rudnicki MA (2011) Satellite cells, the engines of muscle repair. Nat Rev Mol Cell Biol 13(2):127–133
Conboy MJ, Karasov AO, Rando TA (2007) High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol 5(5):e102
Sincennes MC, Brun CE, Lin AYT, Rosembert T, Datzkiw D, Saber J, Ming H, Kawabe YI, Rudnicki MA (2021) Acetylation of PAX7 controls muscle stem cell self-renewal and differentiation potential in mice. Nat Commun 12(1):3253
Saber J, Rudnicki MA (2022) Carm1 and the epigenetic control of stem cell function. Stem Cells Transl Med 11(11):1143–1150
Dumont NA, Wang YX, von Maltzahn J, Pasut A, Bentzinger CF, Brun CE, Rudnicki MA (2015) Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat Med 21(12):1455–1463
Troy A, Cadwallader AB, Fedorov Y, Tyner K, Tanaka KK, Olwin BB (2012) Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38α/β MAPK. Cell Stem Cell 11(4):541–553
Wang YX, Feige P, Brun CE, Hekmatnejad B, Dumont NA, Renaud JM, Faulkes S, Guindon DE, Rudnicki MA (2019) EGFR-Aurka signaling rescues polarity and regeneration defects in dystrophin-deficient muscle stem cells by increasing asymmetric divisions. Cell Stem Cell 24(3):419-432 e416
Almada AE, Wagers AJ (2016) Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol 17(5):267–279
Yue B, Yang H, Wu J, Wang J, Ru W, Cheng J, Huang Y, Lan X, Lei C, Chen H (2022) circSVIL regulates bovine myoblast development by inhibiting STAT1 phosphorylation. Sci China Life Sci 65(2):376–386
Brzoska E, Ciemerych MA, Przewozniak M, Zimowska M (2011) Regulation of muscle stem cells activation: the role of growth factors and extracellular matrix. Vitam Horm 87:239–276
Ciemerych MA, Archacka K, Grabowska I, Przewozniak M (2011) Cell cycle regulation during proliferation and differentiation of mammalian muscle precursor cells. Results Probl Cell Differ 53:473–527
Yeh CJ, Sattler KM, Lepper C (2023) Molecular regulation of satellite cells via intercellular signaling. Gene 858:147172
Mierzejewski B, Archacka K, Grabowska I, Florkowska A, Ciemerych MA, Brzoska E (2020) Human and mouse skeletal muscle stem and progenitor cells in health and disease. Semin Cell Dev Biol 104:93–104
Zammit PS (2017) Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin Cell Dev Biol 72:19–32
Henrot P, Blervaque L, Dupin I, Zysman M, Esteves P, Gouzi F, Hayot M, Pomiès P, Berger P (2023) Cellular interplay in skeletal muscle regeneration and wasting: insights from animal models. J Cachexia Sarcopenia Muscle 14(2):745–757
Zhang L, Wang XH, Wang H, Du J, Mitch WE (2010) Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 21(3):419–427
Wang K, Liu Q, Tang M, Qi G, Qiu C, Huang Y, Yu W, Wang W, Sun H, Ni X et al (2023) Chronic kidney disease-induced muscle atrophy: molecular mechanisms and promising therapies. Biochem Pharmacol 208:115407
Talbert EE, Guttridge DC (2016) Impaired regeneration: a role for the muscle microenvironment in cancer cachexia. Semin Cell Dev Biol 54:82–91
Wang W, Li M, Chen Z, Xu L, Chang M, Wang K, Deng C, Gu Y, Zhou S, Shen Y et al (2022) Biogenesis and function of extracellular vesicles in pathophysiological processes of skeletal muscle atrophy. Biochem Pharmacol 198:114954
Sancho-Muñoz A, Guitart M, Rodríguez DA, Gea J, Martínez-Llorens J, Barreiro E (2021) Deficient muscle regeneration potential in sarcopenic COPD patients: role of satellite cells. J Cell Physiol 236(4):3083–3098
Balnis J, Drake LA, Singer DV, Vincent CE, Korponay TC, D’Armiento J, Lee CG, Elias JA, Singer HA, Jaitovich A (2022) Deaccelerated myogenesis and autophagy in genetically induced pulmonary emphysema. Am J Respir Cell Mol Biol 66(6):623–637
Zhang L, Li M, Wang W, Yu W, Liu H, Wang K, Chang M, Deng C, Ji Y, Shen Y et al (2022) Celecoxib alleviates denervation-induced muscle atrophy by suppressing inflammation and oxidative stress and improving microcirculation. Biochem Pharmacol 203:115186
Shen Y, Li M, Wang K, Qi G, Liu H, Wang W, Ji Y, Chang M, Deng C, Xu F et al (2022) Diabetic muscular atrophy: molecular mechanisms and promising therapies. Front Endocrinol (Lausanne) 13:917113
Wang W, Shen D, Zhang L, Ji Y, Xu L, Chen Z, Shen Y, Gong L, Zhang Q, Shen M et al (2021) SKP-SC-EVs mitigate denervated muscle atrophy by inhibiting oxidative stress and inflammation and improving microcirculation. Antioxidants (Basel, Switzerland) 11(1):66
Huang Z, Zhong L, Zhu J, Xu H, Ma W, Zhang L, Shen Y, Law BY, Ding F, Gu X et al (2020) Inhibition of IL-6/JAK/STAT3 pathway rescues denervation-induced skeletal muscle atrophy. Ann Transl Med 8(24):1681
Shen Y, Zhang R, Xu L, Wan Q, Zhu J, Gu J, Huang Z, Ma W, Shen M, Ding F et al (2019) Microarray analysis of gene expression provides new insights into denervation-induced skeletal muscle atrophy. Front Physiol 10:1298
Qiu J, Fang Q, Xu T, Wu C, Xu L, Wang L, Yang X, Yu S, Zhang Q, Ding F et al (2018) Mechanistic role of reactive oxygen species and therapeutic potential of antioxidants in denervation- or fasting-induced skeletal muscle atrophy. Front Physiol 9:215
Duan D, Goemans N, Takeda S, Mercuri E, Aartsma-Rus A (2021) Duchenne muscular dystrophy. Nat Rev Dis Primers 7(1):13
Chang M, Cai Y, Gao Z, Chen X, Liu B, Zhang C, Yu W, Cao Q, Shen Y, Yao X et al (2023) Duchenne muscular dystrophy: pathogenesis and promising therapies. J Neurol 270(8):3733–3749
Matre PR, Mu X, Wu J, Danila D, Hall MA, Kolonin MG, Darabi R, Huard J (2019) CRISPR/Cas9-based dystrophin restoration reveals a novel role for dystrophin in bioenergetics and stress resistance of muscle progenitors. Stem Cells (Dayton, Ohio) 37(12):1615–1628
Attia M, Maurer M, Robinet M, Le Grand F, Fadel E, Le Panse R, Butler-Browne G, Berrih-Aknin S (2017) Muscle satellite cells are functionally impaired in myasthenia gravis: consequences on muscle regeneration. Acta Neuropathol 134(6):869–888
Hwang AB, Brack AS (2018) Muscle stem cells and aging. Curr Top Dev Biol 126:299–322
Cai Z, Liu D, Yang Y, Xie W, He M, Yu D, Wu Y, Wang X, Xiao W, Li Y (2022) The role and therapeutic potential of stem cells in skeletal muscle in sarcopenia. Stem Cell Res Ther 13(1):28
Lees SJ, Rathbone CR, Booth FW (2006) Age-associated decrease in muscle precursor cell differentiation. Am J Physiol Cell Physiol 290(2):C609–C615
Brack AS, Munoz-Canoves P (2016) The ins and outs of muscle stem cell aging. Skelet Muscle 6:1
Englund DA, Zhang X, Aversa Z, LeBrasseur NK (2021) Skeletal muscle aging, cellular senescence, and senotherapeutics: current knowledge and future directions. Mech Ageing Dev 200:111595
Neelakantan H, Brightwell CR, Graber TG, Maroto R, Wang HL, McHardy SF, Papaconstantinou J, Fry CS, Watowich SJ (2019) Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem Pharmacol 163:481–492
Morena da Silva F, Lim S, Cabrera AR, Schrems ER, Jones RG, Rosa-Caldwell ME, Washington TA, Murach KA, Greene NP (2023) The time-course of cancer cachexia onset reveals biphasic transcriptional disruptions in female skeletal muscle distinct from males. BMC Genomics 24(1):374
Zanders L, Kny M, Hahn A, Schmidt S, Wundersitz S, Todiras M, Lahmann I, Bandyopadhyay A, Wollersheim T, Kaderali L et al (2022) Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting. J Cachexia Sarcopenia Muscle 13(1):713–727
Wan Q, Zhang L, Huang Z, Zhang H, Gu J, Xu H, Yang X, Shen Y, Law BY, Zhu J et al (2020) Aspirin alleviates denervation-induced muscle atrophy via regulating the Sirt1/PGC-1α axis and STAT3 signaling. Ann Transl Med 8(22):1524
Price FD, von Maltzahn J, Bentzinger CF, Dumont NA, Yin H, Chang NC, Wilson DH, Frenette J, Rudnicki MA (2014) Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med 20(10):1174–1181
Domingues-Faria C, Vasson MP, Goncalves-Mendes N, Boirie Y, Walrand S (2016) Skeletal muscle regeneration and impact of aging and nutrition. Ageing Res Rev 26:22–36
Girardi F, Le Grand F (2018) Wnt signaling in skeletal muscle development and regeneration. Prog Mol Biol Transl Sci 153:157–179
Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA (2008) A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2(1):50–59
Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93(1):23–67
Koike H, Manabe I, Oishi Y (2022) Mechanisms of cooperative cell-cell interactions in skeletal muscle regeneration. Inflamm Regener 42(1):48
Wang X, Chen J, Homma ST, Wang Y, Smith GR, Ruf-Zamojski F, Sealfon SC, Zhou L (2023) Diverse effector and regulatory functions of fibro/adipogenic progenitors during skeletal muscle fibrosis in muscular dystrophy. iScience 26(1):105775
Chen W, You W, Valencak TG, Shan T (2022) Bidirectional roles of skeletal muscle fibro-adipogenic progenitors in homeostasis and disease. Ageing Res Rev 80:101682
Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, Alfonsi MV, Nicoletti C, Gatto S, De Bardi M et al (2018) Denervation-activated STAT3-IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis. Nat Cell Biol 20(8):917–927
Leinroth AP, Mirando AJ, Rouse D, Kobayahsi Y, Tata PR, Rueckert HE, Liao Y, Long JT, Chakkalakal JV, Hilton MJ (2022) Identification of distinct non-myogenic skeletal-muscle-resident mesenchymal cell populations. Cell Rep 39(6):110785
Giuliani G, Vumbaca S, Fuoco C, Gargioli C, Giorda E, Massacci G, Palma A, Reggio A, Riccio F, Rosina M et al (2021) SCA-1 micro-heterogeneity in the fate decision of dystrophic fibro/adipogenic progenitors. Cell Death Dis 12(1):122
Oprescu SN, Yue F, Qiu J, Brito LF, Kuang S (2020) Temporal dynamics and heterogeneity of cell populations during skeletal muscle regeneration. iScience 23(4):100993
Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ (2012) Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res 27(5):1004–1017
Malecova B, Gatto S, Etxaniz U, Passafaro M, Cortez A, Nicoletti C, Giordani L, Torcinaro A, De Bardi M, Bicciato S et al (2018) Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Nat Commun 9(1):3670
Farup J, Just J, de Paoli F, Lin L, Jensen JB, Billeskov T, Roman IS, Cömert C, Møller AB, Madaro L et al (2021) Human skeletal muscle CD90(+) fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab 33(11):2201-2214.e2211
Fitzgerald G, Turiel G, Gorski T, Soro-Arnaiz I, Zhang J, Casartelli NC, Masschelein E, Maffiuletti NA, Sutter R, Leunig M et al (2023) MME(+) fibro-adipogenic progenitors are the dominant adipogenic population during fatty infiltration in human skeletal muscle. Commun Biol 6(1):111
Scott RW, Arostegui M, Schweitzer R, Rossi FMV, Underhill TM (2019) Hic1 defines quiescent mesenchymal progenitor subpopulations with distinct functions and fates in skeletal muscle regeneration. Cell Stem Cell 25(6):797-813.e799
Huang X, Yan L, Meng J, Liu N, Zhu S, Jiang Z, Kou S, Feng T, Lin CP, Zhou B et al (2023) Genetic lineage tracing identifies cardiac mesenchymal-to-adipose transition in an arrhythmogenic cardiomyopathy model. Sci China Life Sci 66(1):51–66
Chapman MA, Mukund K, Subramaniam S, Brenner D, Lieber RL (2017) Three distinct cell populations express extracellular matrix proteins and increase in number during skeletal muscle fibrosis. Am J Physiol Cell Physiol 312(2):C131–C143
Rayagiri SS, Ranaldi D, Raven A, Mohamad Azhar NIF, Lefebvre O, Zammit PS, Borycki AG (2018) Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat Commun 9(1):1075
Dagdeviren S, Jung DY, Friedline RH, Noh HL, Kim JH, Patel PR, Tsitsilianos N, Inashima K, Tran DA, Hu X et al (2017) IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J 31(2):701–710
Uezumi A, Ikemoto-Uezumi M, Zhou H, Kurosawa T, Yoshimoto Y, Nakatani M, Hitachi K, Yamaguchi H, Wakatsuki S, Araki T et al (2021) Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia. J Clin Investig 131(1):e139617
Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA (2019) Mesenchymal stromal cells are required for regeneration and homeostatic maintenance of skeletal muscle. Cell Rep 27(7):2029-2035.e2025
Wu J, Ren B, Wang D, Lin H (2022) Regulatory T cells in skeletal muscle repair and regeneration: recent insights. Cell Death Dis 13(8):680
Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D (2016) Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity 44(2):355–367
Reggio A, Rosina M, Krahmer N, Palma A, Petrilli LL, Maiolatesi G, Massacci G, Salvatori I, Valle C, Testa S et al (2020) Metabolic reprogramming of fibro/adipogenic progenitors facilitates muscle regeneration. Life Sci Alliance 3(3):e202000646
Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, Sizzano F, Mashinchian O, Ancel S, Migliavacca E et al (2019) Aging disrupts muscle stem cell function by impairing matricellular WISP1 secretion from fibro-adipogenic progenitors. Cell Stem Cell 24(3):433-446.e437
Zhang W, Liu Y, Zhang H (2021) Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci 11(1):65
Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, Zhang RH, Natarajan A, Nedospasov SA, Rossi FM (2015) Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 21(7):786–794
Xu Z, You W, Chen W, Zhou Y, Nong Q, Valencak TG, Wang Y, Shan T (2021) Single-cell RNA sequencing and lipidomics reveal cell and lipid dynamics of fat infiltration in skeletal muscle. J Cachexia Sarcopenia Muscle 12(1):109–129
Tseng HW, Girard D, Alexander KA, Millard SM, Torossian F, Anginot A, Fleming W, Gueguen J, Goriot ME, Clay D et al (2022) Spinal cord injury reprograms muscle fibroadipogenic progenitors to form heterotopic bones within muscles. Bone Res 10(1):22
Fernandez-Simon E, Suarez-Calvet X, Carrasco-Rozas A, Pinol-Jurado P, Lopez-Fernandez S, Pons G, Bech Serra JJ, de la Torre C, de Luna N, Gallardo E et al (2022) RhoA/ROCK2 signalling is enhanced by PDGF-AA in fibro-adipogenic progenitor cells: implications for Duchenne muscular dystrophy. J Cachexia Sarcopenia Muscle 13(2):1373–1384
Reggio A, Rosina M, Palma A, CerquonePerpetuini A, Petrilli LL, Gargioli C, Fuoco C, Micarelli E, Giuliani G, Cerretani M et al (2020) Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/beta-catenin axis. Cell Death Differ 27(10):2921–2941
Pinol-Jurado P, Suarez-Calvet X, Fernandez-Simon E, Gallardo E, de la Oliva N, Martinez-Muriana A, Gomez-Galvez P, Escudero LM, Perez-Peiro M, Wollin L et al (2018) Nintedanib decreases muscle fibrosis and improves muscle function in a murine model of dystrophinopathy. Cell Death Dis 9(7):776
Ieronimakis N, Hays A, Prasad A, Janebodin K, Duffield JS, Reyes M (2016) PDGFRalpha signalling promotes fibrogenic responses in collagen-producing cells in Duchenne muscular dystrophy. J Pathol 240(4):410–424
Fu C, Chin-Young B, Park G, Guzmán-Seda M, Laudier D, Han WM (2023) WNT7A suppresses adipogenesis of skeletal muscle mesenchymal stem cells and fatty infiltration through the alternative Wnt-Rho-YAP/TAZ signaling axis. Stem Cell Rep 18(4):999–1014
Etienne J, Liu C, Skinner CM, Conboy MJ, Conboy IM (2020) Skeletal muscle as an experimental model of choice to study tissue aging and rejuvenation. Skeletal Muscle 10(1):4
Theret M, Rossi FMV, Contreras O (2021) Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front Physiol 12:673404
Ji Y, Li M, Chang M, Liu R, Qiu J, Wang K, Deng C, Shen Y, Zhu J, Wang W et al (2022) Inflammation: roles in skeletal muscle atrophy. Antioxidants (Basel, Switzerland) 11(9):1686
Parker E, Hamrick MW (2021) Role of fibro-adipogenic progenitor cells in muscle atrophy and musculoskeletal diseases. Curr Opin Pharmacol 58:1–7
Lee DE, McKay LK, Bareja A, Li Y, Khodabukus A, Bursac N, Taylor GA, Baht GS, White JP (2022) Meteorin-like is an injectable peptide that can enhance regeneration in aged muscle through immune-driven fibro/adipogenic progenitor signaling. Nat Commun 13(1):7613
Dessalles CA, Babataheri A, Barakat AI (2021) Pericyte mechanics and mechanobiology. J Cell Sci 134(6):jcs240226
Huang H (2020) Pericyte–endothelial interactions in the retinal microvasculature. Int J Mol Sci 21(19):7413
Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II (2017) Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep 19(9):1902–1916
Hejbol EK, Hajjaj MA, Nielsen O, Schroder HD (2019) Marker expression of interstitial cells in human skeletal muscle: an immunohistochemical study. J Histochem Cytochem 67(11):825–844
Roe C, May LS (2016) A case of leprosy in Malawi. Making the final push towards eradication: a clinical and public health perspective. Infect Dis Poverty 5(1):90
Gautam J, Yao Y (2019) Pericytes in skeletal muscle. Adv Exp Med Biol 1122:59–72
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev 22(16):2298–2314
Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, Messi ML, Mintz A, Delbono O (2014) Type-1 pericytes accumulate after tissue injury and produce collagen in an organ-dependent manner. Stem Cell Res Ther 5(6):122
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2013) Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol 305(11):C1098–C1113
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O (2013) Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res 319(1):45–63
Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S et al (2011) Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2:499
Gautam J, Nirwane A, Yao Y (2017) Laminin differentially regulates the stemness of type I and type II pericytes. Stem Cell Res Ther 8(1):28
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3(3):301–313
Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O (2014) Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci 6:245
Nwadozi E, Haas TL (2021) Emerging roles of pericytes in coordinating skeletal muscle functions: implications and therapeutic potential. Current Tissue Microenvironment Rep 2:29–39
Kostallari E, Baba-Amer Y, Alonso-Martin S, Ngoh P, Relaix F, Lafuste P, Gherardi RK (2015) Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development (Cambridge, England) 142(7):1242–1253
Nwadozi E, Ng A, Strömberg A, Liu HY, Olsson K, Gustafsson T, Haas TL (2019) Leptin is a physiological regulator of skeletal muscle angiogenesis and is locally produced by PDGFRα and PDGFRβ expressing perivascular cells. Angiogenesis 22(1):103–115
Kovacs-Oller T, Ivanova E, Bianchimano P, Sagdullaev BT (2020) The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov 6:39
Fukada K, Kajiya K (2020) Age-related structural alterations of skeletal muscles and associated capillaries. Angiogenesis 23(2):79–82
Cai C, Zambach SA, Grubb S, Tao L, He C, Lind BL, Thomsen KJ, Zhang X, Hald BO, Nielsen RM et al (2023) Impaired dynamics of precapillary sphincters and pericytes at first-order capillaries predict reduced neurovascular function in the aging mouse brain. Nat Aging 3(2):173–184
Baum O, Bernd J, Becker S, Odriozola A, Zuber B, Tschanz SA, Zakrzewicz A, Egginton S, Berkholz J (2020) Structural microangiopathies in skeletal muscle related to systemic vascular pathologies in humans. Front Physiol 11:28
Faulkner A, Tamiato A, Cathery W, Rampin A, Caravaggi CM, Jover E, Allen S, Mellor H, Hauton D, Heather LC et al (2020) Dimethyl-2-oxoglutarate improves redox balance and mitochondrial function in muscle pericytes of individuals with diabetes mellitus. Diabetologia 63(10):2205–2217
Arpino JM, Nong Z, Li F, Yin H, Ghonaim N, Milkovich S, Balint B, O’Neil C, Fraser GM, Goldman D et al (2017) Four-dimensional microvascular analysis reveals that regenerative angiogenesis in ischemic muscle produces a flawed microcirculation. Circ Res 120(9):1453–1465
Murray IR, Baily JE, Chen WCW, Dar A, Gonzalez ZN, Jensen AR, Petrigliano FA, Deb A, Henderson NC (2017) Skeletal and cardiac muscle pericytes: functions and therapeutic potential. Pharmacol Ther 171:65–74
Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD (2015) Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16(1):51–66
Mietus CJ, Lackner TJ, Karvelis PS, Willcockson GT, Shields CM, Lambert NG, Koutakis P, Fuglestad MA, Hernandez H, Haynatzki GR et al (2020) Abnormal microvascular architecture, fibrosis, and pericyte characteristics in the calf muscle of peripheral artery disease patients with claudication and critical limb ischemia. J Clin Med 9(8):2575
Teng YC, Porfírio-Sousa AL, Ribeiro GM, Arend MC, da Silva ML, Chen ES, Rosa DS, Han SW (2021) Analyses of the pericyte transcriptome in ischemic skeletal muscles. Stem Cell Res Ther 12(1):183
Munroe M, Dvoretskiy S, Lopez A, Leong J, Dyle MC, Kong H, Adams CM, Boppart MD (2019) Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization. FASEB J 33(6):7694–7706
Wu YF, Lapp S, Dvoretskiy S, Garcia G, Kim M, Tannehill A, Daniels L, Boppart MD (2022) Optimization of a pericyte therapy to improve muscle recovery after limb immobilization. J Appl Physiol 132(4):1020–1030
Dvoretskiy S, Garg K, Munroe M, Pincu Y, Mahmassani ZS, Coombs C, Blackwell B, Garcia G, Waterstradt G, Lee I et al (2019) The impact of skeletal muscle contraction on CD146(+)Lin(−) pericytes. Am J Physiol Cell Physiol 317(5):C1011–C1024
Yao Y, Norris EH, Mason CE, Strickland S (2016) Laminin regulates PDGFRβ(+) cell stemness and muscle development. Nat Commun 7:11415
Diaz-Manera J, Gallardo E, de Luna N, Navas M, Soria L, Garibaldi M, Rojas-Garcia R, Tonlorenzi R, Cossu G, Illa I (2012) The increase of pericyte population in human neuromuscular disorders supports their role in muscle regeneration in vivo. J Pathol 228(4):544–553
Tedesco FS, Gerli MF, Perani L, Benedetti S, Ungaro F, Cassano M, Antonini S, Tagliafico E, Artusi V, Longa E et al (2012) Transplantation of genetically corrected human iPSC-derived progenitors in mice with limb-girdle muscular dystrophy. Sci Transl Med 4(140):140ra189
Moyle LA, Tedesco FS, Benedetti S (2019) Pericytes in muscular dystrophies. Adv Exp Med Biol 1147:319–344
Cordova G, Negroni E, Cabello-Verrugio C, Mouly V, Trollet C (2018) Combined therapies for Duchenne muscular dystrophy to optimize treatment efficacy. Front Genet 9:114
Mosich GM, Husman R, Shah P, Sharma A, Rezzadeh K, Aderibigbe T, Hu VJ, McClintick DJ, Wu G, Gatto JD et al (2019) Non-fibro-adipogenic pericytes from human embryonic stem cells attenuate degeneration of the chronically injured mouse muscle. JCI Insight 4(24):e125334
Wu YF, De La Toba EA, Dvoretskiy S, Jung R, Kim N, Daniels L, Romanova EV, Drnevich J, Sweedler JV, Boppart MD (2022) Development of a cell-free strategy to recover aged skeletal muscle after disuse. J Physiol 601:5011–5031
Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M (2008) Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26(12):3194–3204
Pannerec A, Formicola L, Besson V, Marazzi G, Sassoon DA (2013) Defining skeletal muscle resident progenitors and their cell fate potentials. Development 140(14):2879–2891
Lewis FC, Cottle BJ, Shone V, Marazzi G, Sassoon D, Tseng CCS, Dankers PYW, Chamuleau SAJ, Nadal-Ginard B, Ellison-Hughes GM (2017) Transplantation of allogeneic PW1(pos)/Pax7(neg) interstitial cells enhance endogenous repair of injured porcine skeletal muscle. JACC Basic Transl Sci 2(6):717–736
Ruchaya PJ, Lewis-McDougall FC, Sornkarn N, Amin S, Grimsdell B, Shaalan A, Gritti G, Soe KT, Clark JE, Ellison-Hughes GM (2022) Transplantation of skeletal muscle-derived Sca-1(+)/PW1(+)/Pax7(−) interstitial cells (PICs) improves cardiac function and attenuates remodeling in mice subjected to myocardial infarction. Cells 11(24):4050
Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR et al (2014) Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344(6184):649–652
Wiesinger A, Li J, Fokkert L, Bakker P, Verkerk AO, Christoffels VM, Boink GJJ, Devalla HD (2022) A single cell transcriptional roadmap of human pacemaker cell differentiation. Elife 11:e76781
Yellamilli A, van Berlo JH (2016) The role of cardiac side population cells in cardiac regeneration. Front Cell Dev Biol 4:102
Klimczak A, Kozlowska U, Kurpisz M (2018) Muscle stem/progenitor cells and mesenchymal stem cells of bone marrow origin for skeletal muscle regeneration in muscular dystrophies. Arch Immunol Ther Exp 66(5):341–354
Meeson AP, Hawke TJ, Graham S, Jiang N, Elterman J, Hutcheson K, Dimaio JM, Gallardo TD, Garry DJ (2004) Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells 22(7):1305–1320
Uezumi A, Ojima K, Fukada S, Ikemoto M, Masuda S, Miyagoe-Suzuki Y, Takeda S (2006) Functional heterogeneity of side population cells in skeletal muscle. Biochem Biophys Res Commun 341(3):864–873
Dight J, Zhao J, Styke C, Khosrotehrani K, Patel J (2022) Resident vascular endothelial progenitor definition and function: the age of reckoning. Angiogenesis 25(1):15–33
Motohashi N, Uezumi A, Yada E, Fukada S, Fukushima K, Imaizumi K, Miyagoe-Suzuki Y, Takeda S (2008) Muscle CD31(−) CD45(−) side population cells promote muscle regeneration by stimulating proliferation and migration of myoblasts. Am J Pathol 173(3):781–791
Doyle MJ, Zhou S, Tanaka KK, Pisconti A, Farina NH, Sorrentino BP, Olwin BB (2011) Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J Cell Biol 195(1):147–163
Penton CM, Thomas-Ahner JM, Johnson EK, McAllister C, Montanaro F (2013) Muscle side population cells from dystrophic or injured muscle adopt a fibro-adipogenic fate. PLoS One 8(1):e54553
Meng J, Muntoni F, Morgan J (2018) CD133+ cells derived from skeletal muscles of Duchenne muscular dystrophy patients have a compromised myogenic and muscle regenerative capability. Stem Cell Res 30:43–52
Meng J, Chun S, Asfahani R, Lochmüller H, Muntoni F, Morgan J (2014) Human skeletal muscle-derived CD133(+) cells form functional satellite cells after intramuscular transplantation in immunodeficient host mice. Mol Ther 22(5):1008–1017
Meng J, Bencze M, Asfahani R, Muntoni F, Morgan JE (2015) The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skeletal Muscle 5:11
Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, Torrente Y, Butler-Browne GS, Mouly V (2009) In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther 17(10):1771–1778
Torrente Y, Belicchi M, Marchesi C, D’Antona G, Cogiamanian F, Pisati F, Gavina M, Giordano R, Tonlorenzi R, Fagiolari G et al (2007) Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant 16(6):563–577
Goloviznina NA, Kyba M (2017) Twist of fate for skeletal muscle mesenchymal cells. Nat Cell Biol 19(3):153–154
Liu N, Garry GA, Li S, Bezprozvannaya S, Sanchez-Ortiz E, Chen B, Shelton JM, Jaichander P, Bassel-Duby R, Olson EN (2017) A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat Cell Biol 19(3):202–213
Min YL, Jaichander P, Sanchez-Ortiz E, Bezprozvannaya S, Malladi VS, Cui M, Wang Z, Bassel-Duby R, Olson EN, Liu N (2018) Identification of a multipotent Twist2-expressing cell population in the adult heart. Proc Natl Acad Sci USA 115(36):E8430–E8439
Li S, Karri D, Sanchez-Ortiz E, Jaichander P, Bassel-Duby R, Liu N, Olson EN (2019) Sema3a-Nrp1 signaling mediates fast-twitch myofiber specificity of Tw2(+) cells. Dev Cell 51(1):89-98.e84
Szondy Z, Al-Zaeed N, Tarban N, Fige É, Garabuczi É, Sarang Z (2022) Involvement of phosphatidylserine receptors in the skeletal muscle regeneration: therapeutic implications. J Cachexia Sarcopenia Muscle 13(4):1961–1973
Gaulton N, Wakelin G, Young LV, Wotherspoon S, Kamal M, Parise G, Nederveen JP, Holwerda A, Verdijk LB, van Loon LJC et al (2022) Twist2-expressing cells reside in human skeletal muscle and are responsive to aging and resistance exercise training. FASEB J 36(12):e22642
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82072160, 32130060, 81901933), the Major Natural Science Research Projects in Universities of Jiangsu Province (No. 20KJA310012), the Natural Science Foundation of Jiangsu Province (Nos. BK20202013, BK20201209), the “QingLan Project” in Jiangsu Universities, the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Nantong Science and Technology Program (Nos. JC22022037, MS22022010, JC12022010).
Author information
Authors and Affiliations
Contributions
HJ, JL and BL drafted the manuscript; HJ, JL, BL, TX, YH, CL, SZ, YG and FX contributed to the analysis of the results, YS, LX and HS reviewed and modified the manuscript. All authors agreed on the final version. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, H., Liu, B., Lin, J. et al. MuSCs and IPCs: roles in skeletal muscle homeostasis, aging and injury. Cell. Mol. Life Sci. 81, 67 (2024). https://doi.org/10.1007/s00018-023-05096-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-05096-w