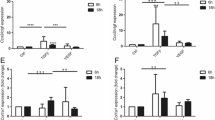
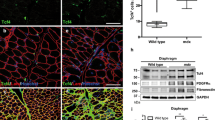
Abstract
Transforming growth factor beta (TGF-β) and connective tissue growth factor (CTGF) have been described to induce the production of extracellular matrix (ECM) proteins and have been reported to be increased in different fibrotic disorders. Skeletal muscle fibrosis is a common feature of Duchenne muscular dystrophy (DMD). The mdx mouse diaphragm is a good model for DMD since it reproduces the muscle degenerative and fibrotic changes. Fibronectin (FN) and proteoglycans (PG) are some of the ECM proteins upregulated in dystrophic conditions. In view of understanding the fibrotic process involved in DMD we have isolated fibroblasts from dystrophic mdx diaphragms. Here we report that regardless of the absence of degenerative myofibers, adult mdx diaphragm fibroblasts show increased levels of FN and condroitin/dermatan sulfate PGs synthesis. Fibroblasts isolated from non fibrotic tissue, such as 1 week old mice diaphragms or skin, do not present elevated FN levels. Furthermore, mdx fibroblast conditioned media is able to stimulate FN synthesis in control fibroblasts. Autocrine TGF-β signaling was unaltered in mdx cells. When control fibroblasts are exposed to TGF-β and CTGF, FN increases as expected. Paradoxically, in mdx cells it decreases in a concentration dependent manner and this decrease is not due to a downregulation of FN synthesis. According to this data we hypothesize that a pathological environment is able to reprogram fibroblasts into an activated phenotype which can be maintained through generations.







Similar content being viewed by others
Abbreviations
- α-SMA:
-
Alpha smooth muscle actin
- CABC :
-
Chondroitinase ABC
- CTGF/CCN2:
-
Connective tissue growth factor
- DMD:
-
Duchenne muscular dystrophy
- ECM:
-
Extracellular matrix
- FN:
-
Fibronectin
- Hase :
-
Heparitinase
- PGs:
-
Proteoglycans
- TGF-β:
-
Transforming growth factor type β
References
Abou-Shady M, Friess H, Zimmermann A, di Mola FF, Guo XZ, Baer HU et al (2000) Connective tissue growth factor in human liver cirrhosis. Liver 20:296–304
Abreu JG, Ketpura NI, Reversade B, De Robertis EM (2002) Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol 4:599–604
Alvarez K, Fadic R, Brandan E (2002) Augmented synthesis and differential localization of heparan sulfate proteoglycans in Duchenne muscular dystrophy. J Cell Biochem 85:703–713
Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL (2002) Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol 157:137–148
Bernasconi P, Di Blasi C, Mora M, Morandi L, Galbiati S, Confalonieri P et al (1999) Transforming growth factor-beta1 and fibrosis in congenital muscular dystrophies. Neuromuscul Disord 9:28–33
Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G et al (2005) Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor beta. Arthritis Rheum 52:1248–1258
Blake DJ (2002) Dystrobrevin dynamics in muscle-cell signalling: a possible target for therapeutic intervention in Duchenne muscular dystrophy. Neuromuscul Disord 12(Suppl 1):S110–S117
Bogdanovich S, Perkins KJ, Krag TO, Khurana TS (2004) Therapeutics for Duchenne muscular dystrophy: current approaches and future directions. J Mol Med 82:102–115
Brandan E, Fuentes ME, Andrade W (1992) Decorin, a chondroitin/dermatan sulfate proteoglycan is under neural control in rat skeletal muscle. J Neurosci Res 32:51–59
Cabello-Verrugio C, Brandan E (2007) A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. J Biol Chem 282:18842–18850
Caceres S, Cuellar C, Casar JC, Garrido J, Schaefer L, Kresse H et al (2000) Synthesis of proteoglycans is augmented in dystrophic mdx mouse skeletal muscle. Eur J Cell Biol 79:173–181
Casar JC, Cabello-Verrugio C, Olguin H, Aldunate R, Inestrosa NC, Brandan E (2004) Heparan sulfate proteoglycans are increased during skeletal muscle regeneration: requirement of syndecan-3 for successful fiber formation. J Cell Sci 117:73–84
Denton CP, Abraham DJ (2001) Transforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesis. Curr Opin Rheumatol 13:505–511
Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X et al (1999) Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. Faseb J 13:1774–1786
Emery AE (2002) Muscular dystrophy into the new millennium. Neuromuscul Disord 12:343–349
Fadic R, Mezzano V, Alvarez K, Cabrera D, Holmgren J, Brandan E (2006) Increase in decorin and biglycan in Duchenne Muscular Dystrophy: role of fibroblasts as cell source of these proteoglycans in the disease. J Cell Mol Med 10(3):758–769
Fisher LW, Stubbs JT 3rd, Young MF (1995) Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand 266:61–65
Flanders KC (2004) Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85:47–64
Fuentealba L, Carey DJ, Brandan E (1999) Antisense inhibition of syndecan-3 expression during skeletal muscle differentiation accelerates myogenesis through a basic fibroblast growth factor-dependent mechanism. J Biol Chem 274:37876–37884
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The Myofibroblast. One Function, Multiple Origins. Am J Pathol 170(6):1807–1816
Hocevar BA, Howe PH (2000) Analysis of TGF-beta-mediated synthesis of extracellular matrix components. Methods Mol Biol 142:55–65
Holmes A, Abraham DJ, Chen Y, Denton C, Shi-Wen X, Black CM et al (2003) Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on sp1. J Biol Chem 278:41728–41733
Kinbara T, Shirasaki F, Kawara S, Inagaki Y, de Crombrugghe B, Takehara K (2002) Transforming growth factor-beta isoforms differently stimulate proalpha2 (I) collagen gene expression during wound healing process in transgenic mice. J Cell Physiol 190:375–381
Kolb M, Margetts PJ, Sime PJ, Gauldie J (2001) Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol 280:L1327–L1334
Lam S, van der Geest RN, Verhagen NA, van Nieuwenhoven FA, Blom IE, Aten J et al (2003) Connective tissue growth factor and igf-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose. Diabetes 52:2975–2983
Leask A, Abraham DJ (2004) TGF-beta signaling and the fibrotic response. Faseb J 18:816–827
Lederfein D, Yaffe D, Nudel U (1993) A housekeeping type promoter, located in the 3′ region of the Duchenne muscular dystrophy gene, controls the expression of Dp71, a major product of the gene. Hum Mol Genet 2:1883–1888
Lopez-Casillas F, Payne H, Andres J, Massague J (1994) Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. J Cell Biol 124:557–568
Lopez-Casillas F, Riquelme C, Perez-Kato Y, Ponce-Castaneda MV, Osses N, Esparza-Lopez J et al (2003) Betaglycan expression is transcriptionally up-regulated during skeletal muscle differentiation. Cloning of murine betaglycan gene promoter and its modulation by MyoD, retinoic acid, and transforming growth factor-beta. J Biol Chem 278:382–390
Melone MA, Peluso G, Galderisi U, Petillo O, Cotrufo R (2000) Increased expression of IGF-binding protein-5 in Duchenne muscular dystrophy (DMD) fibroblasts correlates with the fibroblast-induced downregulation of DMD myoblast growth: an in vitro analysis. J Cell Physiol 185:143–153
Minetti GC, Colussi C, Adami R, Serra C, Mozzetta C, Parente V et al (2006) Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med 12:1147–1150
Nakagawa T, Lan HY, Glushakova O, Zhu HJ, Kang DH, Schreiner GF et al (2005) Role of ERK1/2 and p38 mitogen-activated protein kinases in the regulation of thrombospondin-1 by TGF-beta1 in rat proximal tubular cells and mouse fibroblasts. J Am Soc Nephrol 16:899–904
Niculescu-Duvaz I, Phanish MK, Colville-Nash P, Dockrell ME (2007) The TGFbeta1-induced fibronectin in human renal proximal tubular epithelial cells is p38 MAP kinase dependent and Smad independent. Nephron Exp Nephrol 105:e108–e116
Olguin H, Brandan E (2001) Expression and localization of proteoglycans during limb myogenic activation. Dev Dyn 221:106–115
Osses N, Brandan E (2002) ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol 282:C383–C394
Passerini L, Bernasconi P, Baggi F, Confalonieri P, Cozzi F, Cornelio F et al (2002) Fibrogenic cytokines and extent of fibrosis in muscle of dogs with X-linked golden retriever muscular dystrophy. Neuromuscul Disord 12:828–835
Porter JD, Merriam AP, Leahy P, Gong B, Khanna S (2003) Dissection of temporal gene expression signatures of affected and spared muscle groups in dystrophin-deficient (mdx) mice. Hum Mol Genet 12:1813–1821
Rapaport D, Greenberg DS, Tal M, Yaffe D, Nudel U (1993) Dp71, the nonmuscle product of the Duchenne muscular dystrophy gene is associated with the cell membrane. FEBS Lett 328:197–202
Riquelme C, Larrain J, Schonherr E, Henriquez JP, Kresse H, Brandan E (2001) Antisense inhibition of decorin expression in myoblasts decreases cell responsiveness to transforming growth factor beta and accelerates skeletal muscle differentiation. J Biol Chem 276:3589–3596
Ruperez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M (2003) Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation 108:1499–1505
Salicioni AM, Mizelle KS, Loukinova E, Mikhailenko I, Strickland DK, Gonias SL (2002) The low density lipoprotein receptor-related protein mediates fibronectin catabolism and inhibits fibronectin accumulation on cell surfaces. J Biol Chem 277:16160–16166
Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A et al (2006) Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444:574–579
Santander C, Brandan E (2006) Betaglycan induces TGF-beta signaling in a ligand-independent manner, through activation of the p38 pathway. Cell Signal 18:1492–1491
Sato K, Li Y, Foster W, Fukushima K, Badlani N, Adachi N et al (2003a) Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve 28:365–372
Sato M, Markiewicz M, Yamanaka M, Bielawska A, Mao C, Obeid LM et al (2003b) Modulation of transforming growth factor-beta (TGF-beta) signaling by endogenous sphingolipid mediators. J Biol Chem 278:9276–9282
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Simmons JG, Pucilowska JB, Keku TO, Lund PK (2002) IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol 283:G809–G818
Sottile J, Chandler J (2005) Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell 16:757–768
Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B et al (1991) The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352:536–539
Steinfeld R, Van Den Berghe H, David G (1996) Stimulation of fibroblast growth factor receptor-1 occupancy and signaling by cell surface-associated syndecans and glypican. J Cell Biol 133:405–416
Tidball JG, Wehling-Henricks M (2004) Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res 56:831–841
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3:349–363
van Deutekom JC, van Ommen GJ (2003) Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet 4:774–783
Vial C, Zuniga LM, Cabello-Verrugio C, Canon P, Fadic R, Brandan E (2007) Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol 215:410–421
Wagner KR, McPherron AC, Winik N, Lee SJ (2002) Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52:832–836
Yokoi H, Mukoyama M, Sugawara A, Mori K, Nagae T, Makino H et al (2002) Role of connective tissue growth factor in fibronectin expression and tubulointerstitial fibrosis. Am J Physiol Renal Physiol 282:F933–F942
Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M et al (2007) Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem 282:25852–25863
Acknowledgements
This work was supported in part by grants from FONDAP-Biomedicine No. 13980001, CONICYT AT-24050106 and MDA 3790. The research of E.B. was supported in part by an International Research Scholar grant from the Howard Hughes Medical Institute. V.M. was financed in part by CONICYT and MECESUP. C.V. was financed by CONICYT. The Millenium Institute for Fundamental and Applied Biology is financed in part by the Ministerio de Planificación y Cooperación (Chile).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mezzano, V., Cabrera, D., Vial, C. et al. Constitutively activated dystrophic muscle fibroblasts show a paradoxical response to TGF-β and CTGF/CCN2. J. Cell Commun. Signal. 1, 205–217 (2007). https://doi.org/10.1007/s12079-008-0018-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12079-008-0018-2