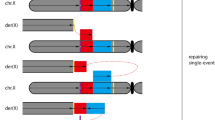
Abstract
Monosomy 1p36 results from a variety of chromosome rearrangements, including terminal deletions, interstitial deletions, derivative chromosomes, and complex rearrangements. Our previous molecular studies on a large cohort of monosomy 1p36 subjects suggest that a significant percentage of terminal deletions of 1p36 are stabilized by the acquisition of telomeric sequences from other chromosome ends, forming derivative chromosomes (i.e., “telomere capture”). However, the molecular mechanism(s) that results in and/or stabilizes terminal deletions of 1p36 by telomere capture is poorly understood. In this report, we have mapped the translocation breakpoints in three subjects with der(1)t(1;1)(p36;q44) chromosomes by fluorescence in situ hybridization (FISH). These results indicate that the breakpoint locations are variable in all three subjects, with no common 1p deletion or 1q translocation breakpoints. In addition, sequence analysis of the 1p and 1q breakpoint-containing clones did not identify homologous sequences or low-copy repeats in the breakpoint regions, suggesting that nonallelic homologous recombination did not play a role in mediating these rearrangements. Microsatellite marker analysis indicates that two of the three derivative chromosomes were formed by intra-chromosomal rearrangements. These data are consistent with a number of recent reports in other model organisms that suggest break-induced replication at the site of a double-strand break may act as a mechanism of telomere capture by generating nonreciprocal translocations from terminally deleted chromosomes. Alternative models are also discussed.

Similar content being viewed by others
References
Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, Schwartz S, Adams MD, Myers EW, Li PW, Eichler EE (2002) Recent segmental duplications in the human genome. Science 297:1003–1007
Ballif BC, Kashork CD, Shaffer LG (2000) FISHing for mechanisms of cytogenetically defined terminal deletions using chromosome-specific subtelomeric probes. Eur J Hum Genet 8:764–770
Ballif BC, Yu W, Shaw CA, Kashork CD, Shaffer LG (2003) Monosomy 1p36 breakpoints suggest pre-meiotic breakage-fusion-bridge cycles are involved in generating terminal deletions. Hum Mol Genet 12:2153–2165
Borgaonkar DS (1984) Chromosomal variation in man, 4th edn. Liss, New York
Bosco G, Haber JE (1998) Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics 150:1037–1047
Brkanac Z, Cody JD, Leach RJ, DuPont BR (1998) Identification of cryptic rearrangements in patients with 18q-deletion syndrome. Am J Hum Genet 62:1500–1506
Catrinel Marinescu R, Johnson EI, Grady D, Chen X-N, Overhauser J (1999) FISH analysis of terminal deletions in patients diagnosed with cri-du-chat syndrome. Clin Genet 56:282–288
Cervantes RB, Lundblad V (2002) Mechanisms of chromosome-end protection. Curr Opin Cell Biol 14:351–356
Chen C, Kolodner RD (1999) Gross chromosomal rearrangements in Saccharomyces cerevisiae replication and recombination defective mutants. Nat Genet 23:81–85
Cooper JP (2000) Telomere transitions in yeast: the end of the chromosome as we know it. Curr Opin Genet Dev 10:169–177
Daniel A, Baker E, Chia N, Haan E, Malfiej P, Hinton L, Clarke N, Adès L, Darmanian A, Callen D (2003) Recombinants of intrachromosomal transposition of subtelomeres in chromosomes 1 and 2: a cause of minute terminal chromosomal imbalances. Am J Med Genet 117:57–64
Difilippantonio MJ, Petersen S, Chen HT, Johnson R, Jasin M, Kanaar R, Ried T, Nussenzweig A (2002) Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J Exp Med 196:469–480
Edelmann L, Spiteri E, Koren K, Puligaal V, Bialer MB, Shanske A, Goldberg R, Morrow, BE (2001) AT-rich palindromes mediate the constitutional t(11;22) translocation. Am J Hum Genet 68:1–13
Emanuel BS, Shaikh TH (2001) Segmental duplications: an ‘expanding’ role in genomic instability and disease. Nat Rev Genet 2:791–800
Fan YS, Jung J, Hamilton B (1999) Small terminal deletion of 1p and duplication of 1q: cytogenetics, FISH studies, and clinical observations at newborn and at 16 years. Am J Med Genet 86:118–123
Flint J, Craddock CF, Villegas A, Bentley DP, Williams HJ, Galanello R, Cao A, Wood WG, Ayyub H, Higgs DR (1994) Healing of broken human chromosomes by the addition of telomeric repeats. Am J Hum Genet 55:505–512
Flint J, Bates GP, Clark K, Dorman A, Willingham D, Roe BA, Micklem G, Higgs DR, Louis EJ (1997) Sequence comparison of human and yeast telomeres identifies structurally distinct subtelomeric domains. Hum Mol Genet 6:1305–1313
Flores-Rozas H, Kolodner RD (2000) Links between replication, recombination and genome instability in eukaryotes. Trends Biochem Sci 25:196–200
Hackett JA, Feldser DM, Greider CW (2001) Telomere dysfunction increases mutation rates and genomic instability. Cell 106:275–286
Heilstedt HA, Ballif BC, Howard LA, Lewis RA, Stal S, Kashork CD, Bacino CA, Shapira SK, Shaffer LG (2003) Physical map of 1p36, placement of breakpoints in monosomy 1p36, and clinical characterization of the syndrome. Am J Hum Genet 72:1200–1212
Inoue K, Lupski JR (2002) Molecular mechanisms for genomic disorders. Annu Rev Genomics Hum Genet 3:199–242
Johnson RD, Jasin M (2001) Double-strand-break-induced homologous recombination in mammalian cells. Biochem Soc Trans 29:196–201
Knight SJ, Flint J (2000) Perfect endings: a review of subtelomeric probes and their use in clinical diagnosis. J Med Genet 37:401–409
Kolodner RD, Putnam CD, Myung K (2002) Maintenance of genome stability in Saccharomyces cerevisiae. Science 297:552–557
Kurahashi H, Shaikh T, Takata M, Toda T, Emanuel BS (2003) The constitutional t(17;22): Another translocation mediated by palindromic AT-rich repeats. Am J Hum Genet 72:733–738
Ledbetter DH (1992) Minireview: Cryptic translocations and telomere integrity. Am J Hum Genet 51:451–456
Malkova A, Ivanov EL, Haber JE (1996) Double-strand break repair in the absence of RAD51 in yeast: a possible role for break-induced DNA replication. Proc Natl Acad Sci USA 93:7131–7136
McEachern MJ, Krauskopf A, Blackburn EH (2000) Telomeres and their control. Annu Rev Genet 34:331–358
Mefford HC, Trask BJ (2002) The complex structure and dynamic evolution of human subtelomeres. Nat Rev Genet 3:91–102
Meltzer PS, Guan X-Y, Trent JM (1993) Telomere capture stabilizes chromosome breakage. Nat Genet 4:252–255
Morrow DM, Connelly C, Hieter P (1997) “Break copy” duplication: a model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147:371–382
Myung K, Datta A, Kolodner RD (2001) Suppression of spontaneous chromosomal rearrangements by S phase checkpoint functions in Saccharomyces cerevisiae. Cell 104:397–408
Neumann AA, Reddel RR (2002) Telomere maintenance and cancer — look, no telomerase. Nat Rev Cancer 2:879–884
Ning Y, Liang JC, Nagarajan L, Schrock E, Ried T (1998) Characterization of 5q deletions by subtelomeric probes and spectral karyotyping. Cancer Genet Cytogenet 103:170–172
Page SL, Shaffer LG (1997) Nonhomologous Robertsonian translocations form predominantly during female meiosis. Nat Genet 15:231–232
Richards RI (2001) Fragile and unstable chromosomes in cancer: causes and consequences. Trends Genet 17:339–345
Shaffer LG, Lupski JR (2000) Molecular mechanisms for constitutional chromosomal rearrangements in humans. Annu Rev Genet 34:297–329
Shaffer LG, McCaskill C, Haller V, Brown JA, Jackson-Cook CK (1993) Further characterization of 19 cases of rea(21q21q) and delineation as isochromosomes or Robertsonian translocations in Down syndrome. Am J Med Genet 47:1218–1222
Shapira SK, McCaskill C, Northrup H, Spikes AS, Elder FF, Sutton VR, Korenberg JR, Greenberg F, Shaffer LG (1997) Chromosome 1p36 deletions: the clinical phenotype and molecular characterization of a common newly delineated syndrome. Am J Hum Genet 61:642–650
Sprung CN, Reynolds GE, Jasin M, Murnane JP (1999) Chromosome healing in mouse embryonic stem cells. Proc Natl Acad Sci USA 96:6781–6786
Stankiewicz P, Lupski JR (2002) Genome architecture, rearrangements and genomic disorders. Trends Genet 18:74–82
Stankiewicz P, Shaw CJ, Dapper JD, Wakui K, Shaffer LG, Withers M, Elizondo L, Park S-S, Lupski JR (2003) Genome architecture catalyzes nonrecurrent chromosomal rearrangements. Am J Hum Genet 72:1101–1116
Varley H, Di S, Scherer SW, Royle NJ (2000) Characterization of terminal deletions at 7q32 and 22q13.3 healed by de novo telomere addition. Am J Hum Genet 67:610–622
Varley H, Pickett HA, Foxon JL, Reddel RR, Royle NJ (2002) Molecular characterization of inter-telomere and intra-telomere mutations in human ALT cells. Nat Genet 30:301–305
Wilkie AOM, Lamb J, Harris PC, Finney RD, Higgs DR (1990) A truncated human chromosome 16 associated with α thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature 346:868–871
Wu Y-Q, Heilstedt HA, Bedell JA, May KM, Starkey DE, McPherson JD, Shapira SK, Shaffer LG (1999) Molecular refinement of the 1p36 deletion syndrome reveals size diversity and a preponderance of maternally derived deletions. Hum Mol Genet 8:313–321
Yu W, Ballif BC, Kashork CD, Heilstedt HA, Howard LA, Cai W-W, White LD, Liu W, Beaudet AL, Bejjani BA, Shaw CD, Shaffer LG (2003) Development of a comparative genomic hybridization microarray and demonstration of its utility with 25 well-characterized 1p36 deletions. Hum Mol Genet 12:2145–2152
Acknowledgements
We thank Cami Knox-Du Bois (Baylor College of Medicine) for the generation of the somatic cell hybrid cell lines, Christopher McCaskill and Leslie Howard (Baylor College of Medicine) for performing the genotyping, and Aaron Theisen (Washington State University Spokane) for his critical editing of the manuscript. We are also grateful to the families who participated in this study and to the clinicians who referred these study subjects. This work was supported by a grant from the NIH National Institute for Child Health and Development P01 HD39420 (LGS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ballif, B.C., Wakui, K., Gajecka, M. et al. Translocation breakpoint mapping and sequence analysis in three monosomy 1p36 subjects with der(1)t(1;1)(p36;q44) suggest mechanisms for telomere capture in stabilizing de novo terminal rearrangements. Hum Genet 114, 198–206 (2004). https://doi.org/10.1007/s00439-003-1029-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-003-1029-y