Abstract
Taenia multiceps (Cestoda: Taeniidae), a worldwide cestode parasite, is emerging as an important helminthic zoonosis due to serious or fatal central nervous system disease commonly known as coenurosis in domestic and wild ruminants including humans. Herein, a fatty acid-binding protein (FABP) gene was identified from transcriptomic data in T. multiceps. This gene, which contains a complete coding sequence, was amplified by reverse-transcriptase polymerase chain reaction. The corresponding protein, which was named TmFABP, had a molecular weight of 14 kDa, and subsequently was recombinantly expressed in Escherichia coli. The fusion protein was purified on Ni-NTA beads (Bio-Rad). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses showed that the purified recombinant protein caused immunogenicity. Immunohistochemical studies showed that TmFABP was expressed at the tegumental level in the protoscolices and in the cells between the body wall and parenchyma layer of the cestode. In sections from gravid proglottids, intense staining was detected in the uterus and eggs. Based on this, TmFABP could be switched on during differentiation of germinative layers to protoscoleces and from metacestodes to adult worms. Taken together, our results already reported for T. multiceps suggest the possibility of TmFABP developing a vaccine to control and prevent coenurosis.
Similar content being viewed by others
Introduction
Coenurosis is a zoonotic disease of artiodactyl animals caused by the larval stage (known as coenurus cerebralis) of Taenia multiceps and Coenurusce cerebralis which parasitize the brain and spinal cord. Coenurosis usually causes encephalitis and neurological symptoms in infected hosts, and sometimes even death (Raether and Hänel 2003; Afonso et al. 2011). Human disease caused by T. multiceps (coenurus), which is distributed worldwide, has been reported in Africa, Europe, Asia and USA (Bohrmann 1990; El-On et al. 2008).
During the life cycle of T. multiceps, the coenurus grows in the reproductive phase, and gradually develops into the adult worm with the maturation of the proglottid starting from the neck. The coenurus phase is the important process in the life cycle, and the development of this parasite is related to nutrition and the physicochemical condition of the host and also to the expression of the correlate regulator gene. Moreover, elucidation of the mechanism of germinal layer development of the protoscolex is very important for preventing teniasis. EgSmadA, EmCBP1, EmCBP2, EgOPN and EmMPK1 may play key roles during parasite development and in parasite–host interactions (Spiliotis et al. 2006; Peng et al. 2006; Camicia et al. 2008; Sako et al. 2011; Li et al. 2011).
Attempts have been made to identify target antigens for the immunodiagnosis and vaccine development for C. cerebralis. The definitive host of T. multiceps is the dog; thus, there is economic significance and biosafety concerns for controlling the release of eggs. In initial vaccine studies, the cyst fluid antigen, the oncosphere antigen and the oncosphere excrete/secrete antigen were found to protect animals against T. multiceps infection (Zhang and Zhang 1991; Hein and Harrison 2005). However, industrial production of a vaccine proved difficult because the source of the native antigens is limited. More recently, the recombinant antigens ‘Tm16’, ‘Tm18’ and ‘45Wat’ were prepared from the onchosphere of T. multiceps, and these were used successfully to protect sheep against infection by this parasite (Gauci et al. 2008; Varcasia et al. 2009; Mu et al. 2011).
Fatty acid-binding proteins (FABP) are low molecular mass (14–15 kDa) members of a protein family that is widely distributed in vertebrates, insects and helminthes (Esteves and Ehrlich 2006), especially lucubrated on the platyhelminths, such as Schistosoma mansoni (Moser et al. 1991), Schistosoma japonica (Yuan et al. 2007), Fasciola hepatica (Timanova-Atanasova et al. 2004; López-Abán et al. 2008), Fasciola gigantica (Nambi et al. 2005; Grams et al. 2006; Allam et al. 2012; Kumar et al. 2012) and Echinococcus spp. (Esteves et al. 1993). Generally, platyhelminth parasites are unable to synthesize most of their own lipids de novo, in particular long chain fatty acids and cholesterol. Consequently, for parasite members in this phylum, FABPs deliver these molecules from the host to a specific destination inside the parasite cells, and this process maintains the metabolism of the entire polypide (Esteves and Ehrlich 2006). Recently, there have been attempts to clone genes encoding EgDf1 from Echinococcus sp., Sj14 from S. japonica, Fh15 from F. hepatica, F. gigantica, FgFABP and Sm14 from S. mansoni. These FABPs are promising candidate antigens for vaccines against these parasites, as indicated by protection from challenge infections (Rodríguez-Pérez et al. 1992; Esteves et al. 1993; Smooker et al. 1997; Estuningsih et al. 1997; Chabalgoity et al. 2000; Yuan et al. 2007; Ramos et al. 2009).
In this present study, we identified the gene encoding FABP from transcriptomic data for T. multiceps, TmFABP, and then studied the expression of TmFABP in the metacestode and adult worm of T. multiceps. Herein, we show that this gene was expressed in the tegumental level of the protoscolex, neck proglottids and eggs of the gravid proglottids. TmFABP could be switched on during differentiation of germinative layers to protoscoleces and from metacestodes to adult worms.
Materials and methods
Parasites and fixation technique
T. multiceps protoscoleces were aspirated from cysts that had been collected from goats that had died of coenurosis on a goat farm in SiChuan province, China. Blood samples were collected from goats which were identified by surgical puncture to be affected by coenurosis. Protoscoleces were washed thoroughly in sterile normal saline and kept in a sterile tube at 4 °C until needed for infection. Some protoscoleces were fixed for histological studies in freshly prepared 4 % (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 1 h and embedded in paraffin or stored in liquid nitrogen for subsequent RNA extraction. Each dog was infected orally with 30 scoleces. The McMaSter flotation technique was used to detect taeniidae eggs in fecal samples from infected dogs, which were used to evaluate patency. The dogs were euthanized with 20 % pentobarbital. T. multiceps adult parasites were removed from the small intestine and stored in 4 % paraformaldehyde in PBS for 1 h for histological studies. All animals were handled in strict accordance with the animal protection law of the People’s Republic of China (a draft of an animal protection law in China was released on September 18, 2009).The protoscoleces and adult parasites were embedded in paraffin and cut with a microtome (Leica, Mod. RM2125RT).
RNA extraction and cDNA synthesis
T. multiceps protoscoleces were frozen in liquid nitrogen and ground to a powder with a pestle and mortar. RNA was extracted using Trizol reagent (Huashun, Shanghai, China) according to the manufacturer’s instructions. The resultant RNA was reverse transcribed using Trizol reagent (Fermentas, Shenzhen, China) according to the manufacturer’s instructions.
Cloning and expression of the mature region of TmFABP in Escherichia coli and purification of the recombinant protein
The cDNA sequence of TmFABP used for designing primers was derived from unigene1299 of the assembled T. multiceps transcriptome dataset with the accession numbers JR918037 in Transcriptome Shotgun Assembly Sequence Database at the National Center for Biotechnology Information (NCBI). The open reading frame of the nucleotide sequence was analysed by Open Reading Frame Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/). The amino acid sequences predicted by primer premier 5.0 were found similarly with previously reported sequences in GenBank by the BLAST network server of NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The forward primer was 5′-CGCCGACCCATGGTGAACGATGCAGAGAAGTA-3′, and the reverse primer was 5′-CCGGCTGAGTTACAGTCCACCTCCTCAATGGT-3′. PCR reactions were performed in a final volume of 50 μL that contained 1× Ex Taq buffer, 2.0 mM MgCl2, 0.2 μM of each primer, 0.2 mM of each dNTP, 5 ng of cDNA and 0.5 units of Ex Taq DNA polymerase (TakaRa, Dalian, China), and the cycling conditions were 5 min at 94 °C followed by 30 cycles of 45 s at 94 °C, 45 s at 56 °C and 45 s at 72 °C. PCR products were separated in a 1.0 % agarose gel, and the DNA fragments were recovered and cloned into the pMD18-T vector (Tiangen, Beijing, China) for sequencing. The mature enzyme region of TmFABP was amplified using primers containing the recognition sequence for the restriction enzyme added to 5′ end. The primers used were 5′-CGCGGATCCATGGTGAACGATGCAGAGAAGTA-3′ (TMF1) and 5′-CCGCTCGAGTTACAGTCCACCTCCTCAATGGT-3′ (TMF2). The PCR reactions were performed using cDNA clones as templates. The PCR products of TmFABP were cloned into the pET32a(+) vector (Novagen) to give fusion proteins with a His tag. The cloned plasmid was transformed into E. coli BL21 (DE3) pLysS strain. Expression of the recombinant protein was induced by the addition of 1 mM isopropyl thiogalactoside (IPTG) to the culture. The expressed recombinant proteins were purified using Ni2+ affinity chromatography (Bio-Rad, Hercules, CA, USA) according the manufacturer’s instructions.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis
The purified protein was treated with SDS sample buffer (62.5 mM Tris–HCl, pH 6.8, 2.0 % SDS, 50 mM dithiothreitol and 10.0 % glycerol) at 100 °C for 5 min and size-separated by 15 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) before being electro-transferred onto a nitrocellulose membrane (Bio-Rad). The sheet was blocked with blocking solution [20 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1.0 % bovine serum albumin (BSA), 0.1 % Tween 20] at room temperature for 2 h, and then incubated for 2 h with serum from goats infected with T. multiceps (diluted 1:200 in phosphate buffer solution) after extensive washing with PBS-T (0.1 % Tween 20). The horse radish peroxidase-conjugated rabbit anti-goat IgG antibody (diluted 1:2000) was used as the secondary antibody, and detection was carried out using diaminobenzidine (DAB) substrates.
Production of polyclonal antibody
Four female rabbits were immunized by hypodermic injection of E. coli-expressed recombinant TmFABP. Each rabbit was immunized with fusion protein emulsified in Freund’s complete adjuvant that included 200 μg of antigen. Two weeks later, the process was repeated for each rabbit, but this time, Freund’s incomplete adjuvant that included 200 μg of antigen was used. A month later, the rabbits were injected with 100 μg of simple fusion recombinant protein via the ear brink vein. Blood serum including polyclonal anti-TmFABP antibodies was collected and purified by HiTrap ProteinA (Bio-Rad, Hercules, CA, USA) for subsequent immunohistochemistry. All animals were handled in strict accordance with the animal protection law of the People’s Republic of China (a draft of an animal protection law in China was released on September 18, 2009).
Immunohistochemistry detection of TmFABP in various life stages of T. multiceps
Tissue sections of T. multiceps, including cysts, protoscoleces and adult worms, were deparaffinized and rehydrated through serial concentrations of ethyl alcohol (100–70 %). Then, sections were treated with 0.01 M citrate buffer (citric acid; pH 6.0) in a microwave oven at 700 W for 5 min. Nonspecific binding was blocked by 0.1 % (w/v) glycine in 0.01 M PBS, and 4 % (w/v) BSA in 0.01 M PBS for 30 min each step. The sections were incubated with rabbit antiserum against anti-TmFABP (diluted 1:2,000) overnight at 4 °C. The sections were washed and then incubated with the goat anti-rabbit IgG (diluted 1:1,000) at room temperature for 1 h in darkness. After three washes in PBS, the sections were incubated with DAB. Finally, the sections were examined and photographed under a Nikon microscope.
Results
Nucleotide sequence analysis
The 402-bp (GenBank accession number GU205473) of the entire TmFABP cDNA sequence contained an initiation codon and open reading frame. The SignalP program did not predict a signal sequence within the full length amino acid sequence of TmFABP. The secondary structure of the protein was predicted with DNAstar. Amino acids 0–35, 50–70, 85–95 and 110–124 of this protein formed alpha regions, while amino acids 35–45, 80–85, 100–110 and 125–133 formed beta regions (Fig. 1). In addition, amino acids 48, 76 and 98 formed a random coil structure. TmFABP shared 97.8 % amino acid identity with the FABP from Taenia solium, greater amino acid identity with Egf2 compared to Egf1 from Echinococcus granulosus but was very different from the amino acid sequence of FABP from Schistosoma japonicum (Fig. 2).
Comparison of the amino acid sequence inferred from the nucleotide sequence of TmFABP with other members of the gene family of fatty acid-binding proteins. Tm fatty acid-binding protein from T. multiceps, Ts fatty acid binding protein from T. solium, Eg1 fatty acid binding protein І from E. granulosus, Eg2 fatty acid-binding protein ІI from E. granulosus, mp2 myelin P2 protein of Homo sapiens, Sm fatty acid binding-protein from S. mansoni
Expression and purification of recombinant TmFABP
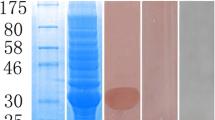
The mature TmFABP gene lacking a signal peptide was inserted into the pET32a vector flanked by BamhI and XohI restriction sites, which allowed the expression of the TmFABP protein coupled with a His-tag at the N-terminus. The protein was expressed successfully at 37 °C, and SDS–PAGE of the protein after purification on Ni-NTA beads (Bio-Rad, Hercules, CA, USA) revealed a band corresponding to the TmFABP at 35 kDa, which is similar to the predicted size of the mature protein fused to a His-tag (34 kDa; Fig. 3). This protein was soluble in the liquid supernatant (not shown).
SDS–PAGE and Western blot of recombinant protein expressed in BL21 (DE3) cells. M protein molecular weight marker, 1 whole cell protein from IPTG-induced cells containing pET-32a(+), 2 whole cell protein from induced cells lacking pET-32a(+), 3 whole cell protein from IPTG-induced cells containing pET-32a-Tm-FABP, 4 whole cell protein from non-induced cells containing pET-32a(+), 5 sample after Ni column collection of purified recombinant protein. A Western blot of expressed rTmFABP probed with serum of Taenia-infected goats. B Western blot of expressed rTmFABP probed with normal goat serum
Western blotting
After SDS–PAGE, the band was stained with Coomassie brilliant blue. TmFABP was blotted onto nitrocellulose membranes and probed with sera from goat infected with T. multiceps. A single 35-kDa band was identified by DAB staining demonstrating that the migrated protein had been transferred successfully (Fig. 3).
Immunolocalization of TmFABP protein in T. multiceps
Puce staining was detected in the cytoplasm of cysts and adult worms. The polyclonal anti-TmFABP serum was employed to investigate the expression of the protoscolex and adult worms. Corresponding control samples were treated with preimmune serum, and these exhibited background levels of staining. The strongest signals appeared at the tegumental level of the protoscolex (Fig. 4), whereas no positive signals were detected in control samples. Positive signals were detected with anti-TmFABP in the germinal layer. The positive staining showed a rectangular or ovoid-shaped distribution in cells of the neck proglottids. The intensity of a reddish reaction product indicated the relatively high concentrations of TmFABP being stored in the cells between the body wall and parenchyma layer of the cestode. Immunohistochemistry of immature proglottids showed that the parenchyma exhibited very low densities of DAB at the tegument, caecal and bladder epithelia. In gravid proglottid sections, positive staining was observed in the granules of the cytoplasm close to the body wall. There was high density staining in the uterus and eggs of the gravid proglottids.
Tm-FABP immunolocalization in T. multiceps. a Control sample of collum segment. b Tm-f1 immunolocalization in collum segment. c Control sample of immature segment. d Tm-f1 immunolocalization in immature segment. e Control sample of maturate segment; f-g, Tm-f1 immunolocalization in maturate segment. h Control sample of C. cerebralis. i-j Tm-f1 immunolocalization in protoscolex
Discussion
Lipids are important biological materials that are indispensable for platyhelminths. Eicosanoids may regulate physiological processes as well as modulating inflammatory and immunological responses in mammals and several invertebrate species including helminthes. The larvae of the cestode Taenia taeniaeformis metabolize linoleic acid rapidly to arachidonic acid and synthesize eicosanoids (Belley and Chadee 1995; Leid and McConnell 1983). However, parasitic platyhelminths are unable to synthesize most of their own lipids de novo; consequently, for parasitic members of this phylum, these molecules must be obtained from the host, and these are delivered to a specific destination in cells of the parasite by FABPs. FABPs function by combining hydrophobic groups to help platyhelminths synthesize most of their own lipids de novo, in particular long chain fatty acids and cholesterol. These multigenic cytosolic proteins are distributed widely across the animal kingdom (Esteves and Ehrlich 2006). In this present study, we cloned and expressed the open reading frame of TmFABP from the protoscolex of T. multiceps. The nucleotide sequence of the open reading frame of TmFABP shares 90 % identity to the T. solium FABP gene (GenBank accession number: HQ259679.1) and 82 % identity to E. granulosus FABP gene (GenBank accession number: AF321117.1) gene. TmFABP shares 97.8 and 92 % amino acid sequence similarity to the FABPs of T. solium and E. granulosus, respectively (GenBank accession numbers: ABB76135.1 and AAK12095.1, respectively). Such high homologues at both the nucleotide and amino acid levels suggest a common conservation for this protein family of genes within cestode parasites as well as a possible common ancestral gene, as already proposed (Hunt et al. 1986; Lee and Yong 2004).
Grams et al. (2006) found that parenchymal cells are rectangular or ovoid-shaped and are distributed in the parenchymal layer of F. gigantica (Grams et al. 2006). The type 2 parenchymal cells contain greatest concentrations of FABP, and these cells are mostly located underneath the muscle layers underlining the tegument and immediately around the organs. It is plausible that F. gigantica Pc2 may specialize in the storage and metabolism of fatty acids and other lipid molecules. The immunohistochemical data in this present study showed that the TmFABP protein, similar to EgDf1, was found in the tegumental level of the protoscolex and in the germinal layer. This may be related to the development of T. multiceps and the detection of putative developmental stimuli from specific hosts, and may be relevant for controlling coenurosis. TmFABP was found in the tegument tubercles and to a lesser extent in the areas between the muscle and body area of adult worms. The cells containing the TmFABP protein were found to branch out to make direct contact with parenchymal cells. T. multiceps probably use FABPs for the uptake and transport of fatty acid molecules to the organs in parenchymal layer to help maintain the survival of the parasite. The results showed that FABP was a critical factor for the growth and maturation of proglottids from the neck of the adult worm. TmFABP was distributed in the muscle layer of T. multiceps. Muscle cells in T. multiceps are similar to those in F. gigantica Pc2 in that they contain FABP and function to store and transport fatty acids and other lipid molecules. TmFABP is mainly distributed in the muscle layer, and this is beneficial for T. multiceps to maintain the polypides by acquiring unsaturated fatty acid from the host. Due to the thick and solid muscular layer of T. multiceps, the lipids, such as eicosanoids transmitted by parenchymal cells, are insufficient to cover energy consumption by the organs. Therefore, it is supposed that cells contain abundant fatty acid-binding proteins which are distributed rarely in the parenchymal layer of T. multiceps. Consequently, it is indicated that parasites can adjust their biological mechanism to adapt to a changing environment.
Recently, the recombinant antigens ‘Tm16 and Tm18’ and ‘45 W’, prepared from the onchosphere of T. multiceps, were used successfully to protect sheep against T. multiceps infections (Gauci et al. 2008; Janssen and Barrett 1995). It is interesting to note that the homologous protein described in T. solium shows immunoprotective activity in animals. TmFABP displayed extensive amino acid sequence homology with FABP2, Sm14 and Sj14 from E. granulosus and FABP from T. solium. Furthermore, the TmFABP gene was expressed differentially in the metacestode and adult worm of T. multiceps, and thus could be used as a correlate regulator gene to monitor the development of the parasites. Combined, these results indicated that TmFABP could show a similar immunoprotective activity against T. multiceps infection, suggesting that TmFABP could be exploited in a vaccine to control and prevent T. multiceps infection.
Conclusions
In this study, we identify the fatty acid-binding protein (FABP) gene from transcriptomic data in T. multiceps and investigate the expression of TmFABP in the metacestode and adult worm of T. multiceps. Our findings show that this gene is expressed in the tegumental level of the protoscolex, neck proglottids and eggs of the gravid proglottids. TmFABP is switched on during differentiation of germinative layers to protoscoleces and from metacestodes to adult worms, which suggests that TmFABP could be exploited in a vaccine to control and prevent T. multiceps infection.
References
Afonso SMS, Mukaratirwa S, Hajovska K, Capece BPS, Cristofol C, Arboic M et al (2011) Prevalence and morphological characteristics of Taenia multiceps cysts (Coenurus cerebralis) from abattoir-slaughtered and experimentally infected goats. J Neuroparasitol 2:1–5. doi:10.4303/jnp/235532
Allam G, Bauomy IR, Hemyeda ZM, Sakran TF (2012) Evaluation of a 14.5 kDa-Fasciola gigantica fatty acid binding protein as a diagnostic antigen for human fascioliasis. Parasitol Res 110:1863–1871
Belley A, Chadee K (1995) Eicosanoid production by parasites from pathogenesis to immunomodulation? Parasitol Today 11:327–334
Bohrmann R (1990) Coenurus in the muscles of a gemsbok (Oryx gazella). Vet Parasitol 36:353–356
Camicia F, Paredes R, Chalar C, Galanti N, Kamenetzky L, Gutierrez A et al (2008) Sequencing, bioinformatic characterization and expression pattern of a putative amino acid transporter from the parasitic cestode Echinococcus granulosus. Gene 411:1–9
Chabalgoity JA, Moreno MI, Carol H, Dougan G, Hormaneche CE (2000) Salmonella typhimurium as a basis for a live oral Echinococcus granulosus vaccine. Vaccine 19:460–469
El-On J, Shelef I, Cagnano E (2008) Taenia multiceps: a rare human cestode infection in Israel. Vet Ital 44:621–631
Esteves A, Ehrlich R (2006) Invertebrate intracellular fatty acid binding proteins. Comp Biochem Physiol C Toxicol Pharmacol 142:262–274
Esteves A, Dallagiovanna B, Ehrlich R (1993) A developmentally regulated gene of Echinococcus granulosus codes for a 15.5-kilodalton polypeptide related to fatty acid binding proteins. Mol Biochem Parasitol 58:215–222
Estuningsih SE, Smooker PM, Wiedosari E, Widjajanti S, Vaiano S, Partoutomo S et al (1997) Evaluation of antigens of Fasciola gigantica as vaccines against tropical fasciolosis in cattle. Int J Parasitol 27:1419–1428
Gauci C, Vural G, Cncel T, Oncel T, Varcasia A, Damina V et al (2008) Vaccination with recombinant oncosphere antigens reduces the susceptibility of sheep to infection with Taenia multiceps. Int J Parasitol 38:1041–1050
Grams HR, Viyanant V, Pankao V, Sirisriro A, Meepool A, Kangwanrangsan N et al (2006) Classification of the parenchymal cells in Fasciola gigantica based on ultrastructure and their expression of fatty acid binding proteins (FABPs). Vet Parasitol 142:281–292
Hein W, Harrison G (2005) Vaccines against veterinary helminthes. Vet Parasitol 132:217–222
Hunt RC, Ro JHS, Dobson DE, Min HY, Spiegelman BM (1986) Adipocyte P2 gene: developmental expression and homology of 5′-flanking sequence among fat cell-specific genes. Proc Natl Acad Sci USA 83:3786–3790
Janssen D, Barrett J (1995) A novel lipid-binding protein from the cestode Moniezia expansa. Biochem J 311:49
Kumar N, Anju V, Gaurav N, Chandra D, Samanta S, Gupta SC et al (2012) Vaccination of buffaloes with Fasciola gigantica recombinant glutathione S-transferase and fatty acid binding protein. Parasitol Res 110:419–426
Lee JS, Yong TS (2004) Expression and cross-species reactivity of fatty acid-binding protein of Clonorchis sinensis. Parasitol Res 93:339–343
Leid R, McConnell L (1983) Thromboxane A2 generation by the larval cestode, Taenia taeniaeformis. Clin Immunol Immunopathol 28(1):67–76
Li J, Zhang C, Lv G, Wang J, Wei X, Lin R, Yan G (2011) Cloning and preliminary characterization of a SmadA gene from Echinococcus granulosus. Chin J Anim Vet Sci 12:1756–1762
López-Abán J, Nogal-Ruiz J, Vicente B, Morrondo P, Diez B, Hillyer GV (2008) The addition of a new immunomodulator with the adjuvant adaptation ADAD system using fatty acid binding proteins increases the protection against Fasciola hepatica. Vet Parasitol 153:176–181
Moser D, Tendler M, Griffiths G, Klinkert MQ (1991) A 14-kDa Schistosoma mansoni polypeptide is homologous to a gene family of fatty acid binding proteins. J Biol Chem 266:8447–8454
Mu J, Yang YD, Yang GY, Wang YW, An XX et al (2011) Prokaryotic expression and immunogenicity analysis of Tm45W gene from Taenia multiceps oncospheres. Chin Vet Sci 41:167–172
Nambi PA, Yadav SC, Raina OK, Sriveny D, Saini M (2005) Vaccination of buffaloes with Fasciola gigantica recombinant fatty acid binding protein. Parasitol Res 97:129–135
Peng XY, Li JH, Wu XW, Zhang SJ, Niu JH, Chen XP, Yao J, Sun H (2006) Detection of Osteopontin in the pericyst of human hepatic Echinococcus granulosus. Acta Trop 100:163–171
Raether W, Hänel H (2003) Epidemiology, clinical manifestations and diagnosis of zoonotic cestode infections: an update. Parasitol Res 91:412–438
Ramos CRR, Spisni A, Oyama JS, Sforca ML, Ramos HR, Vilar MM et al (2009) Stability improvement of the fatty acid binding protein Sm14 from S. mansoni by Cys replacement: structural and functional characterization of a vaccine candidate. Biochim Biophys Acta 1794:655–662
Rodríguez-Pérez J, García-Blanco MA, Hillyer GV, Medina R (1992) Fasciola hepatica: molecular cloning, nucleotide sequence, and expression of a gene encoding a polypeptide homologous to a Schistosoma mansoni fatty acid-binding protein. Exp Parasitol 74:400–407
Sako Y, Nakaya K, Ito A (2011) Echinococcus multilocularis: identification and functional characterization of cathepsin B-like peptidases from metacestode. Exp Parasitol 127:693–701
Smooker PM, Hickford D, Vaiano SA, Spithill TW (1997) Isolation, cloning, and expression of fatty-acid binding proteins from Fasciola gigantica. Exp Parasitol 85:86–91
Spiliotis M, Konrad C, Gelmedin V, Tappe D, Bruckenr S, Mosch HU et al (2006) Characterisation of EmMPK1, an ERK-like MAP kinase from Echinococcus multilocularis which is activated in response to human epidermal growth factor. Int J Parasitol 36:1097–1112
Timanova-Atanasova A, Jordanova R, Radoslavov G, Deevska G, Bankov L, Barrett J (2004) A native 13-kDa fatty acid binding protein from the liver fluke Fasciola hepatica. Biochim Biophys Acta 1674:200–204
Varcasia A, Tosciri G, Coccone G, Pipia AP, Garippa G, Damien V et al (2009) Preliminary field trial of a vaccine against coenurosis caused by Taenia multiceps. Vet Parasitol 162:285–289
Yuan H, You-en S, Long-jiang Y, Xiaohua Z, Liuzhe L, Cash M et al (2007) Studies on the protective immunity of Schistosoma japonicum bivalent DNA vaccine encoding Sj23 and Sj14. Exp Parasitol 115:379–386
Zhang W, Zhang W (1991) Metabolin from Taenia multiceps oncospheres against coenurosis of Mutton. Chin J Vet 21:35–36
Acknowledgments
We are grateful to Dr. Sanjie Cao (College of Veterinary Medicine, Sichuan Agricultural University, China) for providing instrument and his constructive suggestions. This project was supported by a grant from the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT; grant no. IRT0848).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nie, Hm., Xie, Y., Fu, Y. et al. Cloning and characterization of the fatty acid-binding protein gene from the protoscolex of Taenia multiceps . Parasitol Res 112, 1833–1839 (2013). https://doi.org/10.1007/s00436-013-3328-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3328-0