Abstract
The aim of the present study was to evaluate the efficiency of 14.5 kDa-Fasciola gigantica fatty acid binding protein (FABP) as a diagnostic antigen for human fascioliasis. 14.5 kDa FABP was isolated from the crude extract of adult F. gigantica worms by ion exchange chromatography followed by gel filtration chromatography and then analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis under reducing condition. Anti-FABP IgG polyclonal antibody (pAb) was generated in rabbits and purified by using sequential use of ammonium sulfate, caprylic acid, and then ion exchange chromatography. Conjugation of purified rabbit anti-FABP IgG with horse reddish peroxidase (HRP) was conducted and used in detecting the coproantigen in the stool and the circulating Fasciola antigen (CA) in the sera of Fasciola-infected patients using sandwich enzyme-linked immunosorbent assay (ELISA). The sensitivities of sandwich ELISA test were 96.43% and 94.74%, while the test specificities were 94.87% and 84.62% for the detection of coproantigen and CA, respectively. The parasitological diagnosis using the Kato–Katz technique revealed 64.29% sensitivity with 100% specificity. The diagnostic efficacy of sandwich ELISA was 95.52% for coproantigen and 87.93% for CA detection. In contrast, the diagnostic efficacy of Kato–Katz technique was 85.07%. It was concluded that 14.5 kDa FABP represented a valuable antigen for the immunodiagnosis of human fascioliasis using sandwich ELISA.
Similar content being viewed by others
Introduction
Fascioliasis is an important public health problem in many parts of the world. Fasciola hepatica and Fasciola gigantica are the most common causes of fascioliasis. F. hepatica is present in Europe, Africa and America, while F. gigantica, the causative agent of tropical fascioliasis in domestic ruminants in Asia and Africa, inflicts an annual economic loss of over US$3 billion to the livestock sector (Spithill et al. 1999; WHO 2004). Humans become infected by eating uncooked and usually unwashed aquatic vegetables harboring encysted larval parasites (Mas-Coma et al. 1999). An estimated range from 2.4 to 17 million people infected with fascioliasis worldwide (Esteban et al. 2003; Mas-Coma 2004, 2005).
The parasitological (coprological) diagnosis of fascioliasis is based on the identification of eggs in the stool. However, the eggs may be absent or scarcely voided at irregular intervals (Hillyer 1988). On the other hand, the eggs may be transiently present in stool after ingestion of raw or uncooked liver from infected animals. Moreover, the symptoms of fascioliasis may be present for several weeks before the eggs are recovered in the stool. Accordingly, the serologic tests represent alternative approaches for the early diagnosis of fascioliasis. However, cross-reactions with other helminthes antigens may confuse the interpretation of the results (Haseeb et al. 2002).
Many immunological techniques have been developed over the past years for diagnosis of parasitic infections in a trial to replace the classical parasitological techniques which are time-consuming and usually lack sensitivity and reproducibility with a possibility of miss diagnosis (García-Rodriguez et al. 1985). Enzyme-linked immunosorbent assay (ELISA) and its different modification systems represent the most common immunodiagnostic methods for fascioliasis (Silvana et al. 2001). Antigen detection assays are of prime importance in the immunodiagnosis, as the detection of circulating Fasciola antigens and coproantigens can denote active infections (Espino et al. 1990, 1992; Abdel-Rahman et al. 1999).
Several antigens are needed for the development of efficient diagnostic methods and/or vaccine preparation. Fasciola antigens are mostly released from rapid turn-over of the external covering of the tegument. It has been documented that several antigens such as fatty acid binding proteins (FABPs) and cathepsin L1 protease were useful diagnostic molecules (O’Neill et al. 1998; Hillyer 1999; Raina et al. 2006). In Fasciola, FABPs are the carrier proteins that help in the uptake of fatty acids from the hosts’ fluids (Ockner 1990). Several attempts have been made to utilize both native and recombinant FABP in both immunodiagnosis and vaccine development for fascioliasis (Estuningsih et al. 1997; Ramajo et al. 2001; Sirisriro et al. 2002; Nambi et al. 2005). The aim of the present study was to isolate, purify, and estimate the diagnostic potential of F. gigantica-derived 14.5 kDa FABP in immunodiagnosis of human fascioliasis.
Materials and methods
Animals
Parasite collection
Adult live F. gigantica flukes were collected from the liver and the bile ducts of naturally infected water buffaloes (Bubalus bubalis) at local abattoir (Moneeb, Giza, Egypt). Worms were extensively washed with chilled physiological saline and phosphate buffered saline (PBS, pH 7.2).
Rabbits
Three parasites free, 8-month-old female New Zealand white rabbits (about 2 kg in weight) were used for mono-specific anti-FABP antibody production. Rabbits were kept under standard laboratory conditions at 21°C, 45–55% humidity, filtered drinking water, and standard diet (24% protein and 4% fat).
Preparation and purification of 14.5 kDa FABP
Adult live F. gigantica flukes were homogenized in 20 mM Tris–HCl buffer (BDH Chemicals, England) containing 5 mM PMSF as a protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA). The parasite homogenate was centrifuged at 10,000 × g for 15 min. at 4°C. The supernatant was collected and the protein content was determined according to Bradford (1976). Crude extract was subjected to ammonium sulfate precipitation method according to Nowotny (1979). FABP was purified from the crude extract by a combination of ion exchange chromatography on DEAE-Sephadex A-50 and gel filtration using Sephacryl HR-100. Absorbance of each fraction was measured at 280 nm and the purity of the produced protein was assayed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions according to Laemmli (1970).
Production and purification of anti-FABP polyclonal antibody
Immunization of rabbits
Before immunization, blood samples were collected from each rabbit and examined by indirect ELISA for cross-reaction with soluble adult worm protein (SWAP) of F. gigantica, Schistosoma mansoni, Schistosoma haematobium, and Hymenolepis nana [Biological supply center, Theodore Bilharz Research Institute (TBRI), Giza, Egypt] to ensure that the rabbits were free from any previous parasitic infection. Any rabbit showed a positive cross-reaction with any tested antigen was excluded from the experiment.
F. gigantica FABP (1 mg) was mixed with an equal volume of the complete Freund’s adjuvant and injected intramuscularly into each rabbit according to Guobadia and Fagbemi (1997). Booster doses (0.5 mg of FABP was mixed with an equal volume of the incomplete Freund’s adjuvant) were administered at the 2nd and 3rd week post the 1st immunization according to Fagbemi et al. (1995). Sera were collected 4 days after the last injection from the ear vein to measure the titer of the produced antibodies. Upon getting high Ab titers, the animal was scarified, the blood was collected, and the sera were prepared and stored at −80°C till used.
Purification and labeling of anti-FABP IgG
IgG purification was based on sequential use of ammonium sulfate precipitation method (Nowotny 1979), caprylic acid purification method (Mckinney and Parkinson 1987), and DEAE-Sephadex A-50 ion exchange chromatography method (Sheehan and FitzGerald 1996). After purification, the protein content was estimated by Bio-Rad protein assay (Bradford 1976) and the purity of IgG was identified by SDS-PAGE (Laemmli 1970). Anti-FABP IgG were conjugated with horseradish peroxidase (HEP) using periodate method according to Tijssen and Kurstak (1984).
The reactivity of produced IgG to 14.5 kDa FABP of F. gigantica and other parasites antigens by using indirect ELISA
This method was performed according to Engvall and Perlman (1971) with some modifications. Briefly, 100 μl/well of either 14.5 kDa FABP of F. gigantica or one of the other helminthes (SWAP of S. mansoni, SWAP of S. haematobium or SWAP of H. nana) antigens at a concentration of 30 μg/ml in 0.06 M carbonate buffer (pH 9.6) were added to 96 microtiter plate and incubated overnight at room temperature. The plates were washed three times with washing buffer (0.1 M PBS/T20, pH 7.4), then blocked with 200 μl/well of 0.1 BSA in 0.1 M PBS for 1 h at 37°C. After three times washing, 100 μl of serially diluted anti-FABP IgG (1/25, 1/50, 1/100, 1/200, 1/400, 1/3200) were applied to wells and incubated for 2 h at 37°C. Then plates were washed three times, and 100 μl of anti-rabbit IgG peroxidase conjugate (Sigma–Aldrich) diluted in washing buffer (1/1000) was dispensed into each well. The plates were incubated for 1 h at 37°C and then washed five times with washing buffer followed by adding 100 μl of OPD substrate working solution to each well. The plates were placed in the dark at room temperature for 30 min. 50 μl/well of 2 N H2SO4 was added to each well to stop the enzyme-substrate reaction. The absorbance was measured at 492 nm using ELISA reader (Bio-Rad microplate reader).
Population study
Collection of samples from human subjects
Stool and blood samples were collected from 28 Fasciola-infected patients, 10 patients infected with S. mansoni, 9 patients infected with S. haematobium, 9 patients infected with H. nana, and 11 patients infected with Ancylostoma spp. All samples were collected from outpatient clinics at TBRI hospital. In addition, ten individuals of the medical staff at TBRI were used as parasite free-healthy negative control. Sera were separated, aliquoted, and kept at −80°C until used. Stool samples were examined at the same day of collection.
Parasitological examination
Fecal samples were collected in clean, wide-mouthed containers with tight-fitting covers. Coprological examination of fecal samples was carried out microscopically by Kato–Katz technique according to Engels et al. (1997).
Standardization of anti-FABP IgG using sandwich ELISA
The microtitre plates (Dynatech) were coated with 100 μl of purified polyclonal IgG with different concentration (2.5, 5, 10, 20, and 30 μg/ml) in 0.06 M carbonate buffer (pH 9.6) and incubated overnight at room temperature. The plates were washed three times and blocked with 1% BSA for 2 h at 37°C. After five times washing, 100 μl of 14.5 kDa FABP in different concentration (0.1, 1, and 10 μg /ml), 100 μl of pooled positive sera and 100 μl of negative sera were added separately and incubated for 2 h at 37°C. After the plates washing, 100 μl of peroxidase-conjugated polyclonal IgG with different dilution (1/50, 1/100, 1/250, 1/500, 1/1000) were added, and plates were incubated for 1 h at 37°C. After five times washing, 100 μl of OPD substrate solution was added to each well, and the plates were placed in the dark at room temperature for 30 min. Then 50 ml/well of 2 N H2SO4 was added and the absorbance was measured at 492 nm using ELISA reader.
Standard curve for FABP detecting limit using anti-FABP IgG with sandwich ELISA
ELISA plates were coated with 100 μl/well of 10 μg/ml purified anti-FABP IgG in carbonate buffer. The plates were washed three times and blocked with 1% BSA for 2 h at 37°C. The plates were washed three times. Serial dilutions of FABP antigens (100 μl, starting from 10 to 0.0001 μg/ml in PBS/T20, pH 7.4) were added to each well and incubated for 2 h at 37°C. After the plates washing, 100 μl of peroxidase-conjugated anti-FABP IgG of dilution 1/250 was dispensed into each well and the plates were incubated for 1 h at 37°C. The assay was completed as mentioned above with ELISA procedure.
Detection of circulating antigen and coproantigen in collected samples by sandwich ELISA
For detection of the circulating Fasciola antigen in sera, the microtitration plates were coated with 100 μl/well of 10 μg/ml purified anti-FABP IgG and incubated overnight at room temperature. The plates were washed three times, blocked by 200 μl of 1% BSA and incubated for 2 h at 37°C. Then the plates were washed three times, and 100 μl of serum samples was added to each well and incubated for 2 h at 37°C. After the plates washing, 100 μl of peroxidase-conjugated anti-FABP IgG of dilution 1/250 was added and plates were incubated for 1 h at room temperature. The assay was completed as mentioned previously in sandwich ELISA procedure.
For detection of coproantigen, stool was diluted in a ratio of 1:3 with saline and the exact method mentioned in serum ELISA was applied to detect the presence of coproantigen.
Key features in reliability of test results
Test specificity and sensitivity can be selected and adjusted to meet the needs of a clinician for the diagnosis and monitoring of a disease. This may be accomplished by changing the selection of the reference value (i.e., cutoff or upper limit of normal) for a particular test (Zane 2001). Diagnostic sensitivity of a method was calculated according to the following formula: Sensitivity (%) = (No. of true positive results × 100)/(No. of positive results + No. of false negative results). Diagnostic specificity of a method was calculated according to the following formula: Specificity (%) = (No. of true negative results × 100)/(No. of negative results + No. of false positive results). Efficiency of a particular method is a calculated percentage of individuals that are correctly classified as positive (diseased) or negative (nondiseased) for a particular disease (Zane 2001), calculated as follows: Efficiency (%) = (No. of true positives + No. of true negatives) × 100/(No. of true positives + No. of false positives + No. of false negatives + No. of true negatives).
Statistical analysis
The data were presented as mean (X) ± standard deviation (SD). The mean of the groups were compared by analysis of variance (Snedecor and Cochran 1981) using either Student’s t-test or ANOVA. The data were considered significant if p ≤ 0.05. All statistical analyses were performed using the SPSS program.
Results
Purification of FABP from crude extracts of F. gigantica adult worms
Protein content of crude extract that obtained from adult F. gigantica worms was 20 mg/ml. Crude extracts of F. gigantica was subjected to 50% ammonium sulfate saturation. The protein content was 5 mg/ml in the supernatant after saturation.
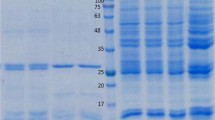
DEAE chromatography is an effective method for separating proteins based on their charge. Two peaks were detected after purification by DEAE Sephadex A-50 ion exchange column chromatography. The first peak was found to contain FABP with maximum optical density (OD) value at 1.9 for fraction number 6, and represented by single band at 14.5 kDa with traces of other proteins at 29, 34, 50, and 80 kDa (Fig. 1). The protein content of these fractions was 2.5 mg/ml. The second peak was found to contain 17 kDa protein with maximum OD value 0.94 for fraction number 4 (Fig. 1). The protein content after purification by DEAE Sephadex A-50 ion exchange column chromatography was 2.5 mg/ml.
12% SDS-PAGE analysis profile under reducing conditions. Lane 1: Molecular mass markers. Lane 2: Crude extract protein was separated into several bands ranging from 12 to 117 kDa, the major bands being at 75, 50, 34, 29, 17, and 14.5 kDa. Lane 3: FABP fraction after DEAE Sephadex A-50 chromatography (fractions of peak1). Lane 4: Fractions of peak 2 washed from DEAE Sephadex A-50 column. Lane 5: Purified FAPB eluted from Sephacryl HR-100 column. The gel was stained with 0.1% Coomassie blue
The fractions of the first peak obtained after purification of post saturation supernatant by DEAE Sephadex A-50 ion exchange column were further purified by Sephacryl HR-100 gel filtration column chromatography. Eluted FABP was represented by a single peak with maximum OD value 1.2 for fraction number 6. The purity of the eluted FAPB in the collected fraction was assayed by 12% SDS PAGE. The purified FABP was represented by a single band at 14.5 kDa (Fig. 1). The yield of FABP by these methods was 1.5 mg/ml from the starting protein content in crude extract of 20 mg/ml.
Production and purification of anti-FABP polyclonal IgG
The antibody level reached the highest titer (2.75 OD) after the 2nd booster dose. The total protein content of crude rabbit serum containing anti-Fasciola antibody was 10.2 mg/ml. The yield of purified anti-FABP IgG antibody following each purification step was determined by assessment of the protein content.
Using 50% ammonium sulfate precipitation method, the protein content was 4.7 mg/ml, while following 7% caprylic acid precipitation method, the content dropped to 2.3 mg/ml. On the other hand, the protein content of highly purified anti-FABP IgG antibody that subjected to ion exchange chromatography method was 1.1 mg/ml.
Eluted IgG was represented by a single peak with maximum OD value at 2.86 for fraction number ten following purification by DEAE Sephadex A-50 ion exchange column chromatography. Analysis of 50% ammonium sulfate-precipitated proteins by 12.5% SDS-PAGE under reducing condition showed that the precipitated proteins appeared in several bands. Purity of the IgG after each step was assayed by 12.5% SDS-PAGE under the reducing condition. Purified IgG was represented by H- and L-chain at 53 and 31 kDa, respectively. IgG appeared free from any other proteins (Fig. 2).
12.5 % SDS-PAGE of anti-FABP IgG pAb. Lane 1: Mw standard proteins. Lane 2: Crude anti-FABP IgG. Lane 3: precipitated proteins after 50% ammonium sulfate treatment. Lane 4: Purified anti-FABP IgG after 7% caprylic acid treatment and following purification by DEAE Sephadex A-50 ion exchange column chromatography. The gel was stained with 0.1% Coomassie blue
Testing the reactivity and specificity of anti-FABP IgG against F. gigantica FABP by indirect ELISA
The reactivity of anti-FABP IgG against 14.5 kDa FABP of F. gigantica and SWAP of other parasites (S. mansoni, S. haematobium, and H. nana) was determined by indirect ELISA. Anti-FABP IgG at 1/250 dilution gave the strongest reactivity to FABP of F. gigantica. The mean OD of FABP was 2.760 ± 0.127 compared to 0.492 ± 0.129, 0.201 ± 0.112, and 0.224 ± 0.092 of the SWAP of S. mansoni, S. haematobium, and H. nana, respectively.
Stool examination of Fasciola-infected patients using Kato-Katz technique
Out of 28 patients, 18 were found to be true positive to Fasciola ova, while the other ten patients were found to be false negative (64.29% sensitivity). Mean eggs/g stool was 26.17 ± 13.76.
Standardization of anti-FABP polyclonal IgG using sandwich ELISA
Either the purified anti-FABP IgG or anti-IgG peroxidase conjugate (10 μg/ml) was found to be the optimum concentration that resulted in highest test sensitivity.
F. gigantica FABP detecting limit using IgG and sandwich ELISA
The antigen (F. gigantica FABP) was serially diluted from 10 μg/ml to 0.1 ng/ml in 0.1 M PBS/T20 (pH 7.4). Different concentrations of F. gigantica FABP were plotted against their corresponding OD reading to produce standard curve. The lower detection limit of the assay was 1 ng/ml of F. gigantica FABP using anti-FABP polyclonal IgG.
Detection of coproantigen in stool samples of infected individuals
As depicted in Table 1, the mean OD value of the patients infected with Fasciola was significantly (P < 0.01) higher than both negative control and individuals infected with other parasites. One out of 28 Fasciola-infected patients gave false negative result. Therefore, the sensitivity of the assay was 96.43%. All the ten negative controls were below the cutoff value (0.351). The specificity was 94.87%, where 2 out of 39 patients were found infected with other parasites. S. mansoni-infected patients (10%) and patients with ancylostomiasis (9.09%) showed positive reaction, whereas patients infected with S. haematobium and H. nana did not show any reactivity.
Detection of FABP in serum of patients
As shown in Table 2, the mean OD value of Fasciola-infected group was significantly (P < 0.01) higher than both negative control group and patients infected with other parasites (S. mansoni, S. haematobium, H. nana, and Ancylostoma spp.). One out of 19 Fasciola-infected patients showed negative result, and the sensitivity of the assay was 94.74%. All healthy control groups were below the cutoff value (0.397). However, 6 out of 39 patients infected with other parasites were above the cutoff value giving 84.62% specificity. The highest positivity was observed with schistosomiasis mansoni in two cases (20% positivity), followed by ancylostomiasis (18.18% positivity), while the lowest positivity was H. nana (11.11% positivity) and schistosomiasis haematobium (11.11% positivity).
Incidence of positivity for coproantigen and circulating FABP antigen
The data of sandwich ELISA with coproantigen in stool of Fasciola-infected human showed that 27 out of 28 cases were positive (96.43%). However, the data of sandwich ELISA with circulating FABP antigen in serum showed 18 out of 19 cases of infected group were positive (94.74%, Table 3).
In the other groups, by using sandwich ELISA for coproantigen detection, the highest positivity was observed with schistosomiasis mansoni (10%), followed by ancylostomiasis (9.09%). However, schistosomiasis haematobium and H. nana was completely negative. While in sera, the highest positivity was observed with schistosomiasis mansoni (20%), followed by ancylostomiasis (18.18%); however, S. haematobium and H. nana showed the lowest positivity (11.11%, Table 3).
The sensitivity, specificity, and the diagnostic efficacy of parasitological analysis and sandwich ELISA for diagnosis of human fascioliasis
The sensitivity, specificity, and the diagnostic efficacy of parasitological analysis were 64.29%, 100%, and 85.07%, respectively. On the other hand, the sensitivity, specificity and the diagnostic efficacy of sandwich ELISA for coproantigen detection were 96.43%, 94.87%, and 95.52%, respectively. However, the sensitivity, specificity, and diagnostic efficacy of sandwich ELISA for circulating FABP antigen detection were 94.74%, 84.62%, and 87.93%, respectively (Table 4).
Discussion
Direct parasitological method for diagnosis of human fascioliasis usually lacks the sensitivity and reproducibility and not reliable (Carnevale et al. 2001). The data of the present study showed that the sensitivity of coprological examination was 64.29%. These data were parallel to that achieved by Anderson et al. (1999), who indicated that the sensitivity of the egg-counting method was 66.7%, and the specificity was 100%, with overall accuracy of 73.9%. Similarly, Haseeb et al. (2002) reported that the eggs might be present in a small number at irregular intervals; hence, it is difficult to be found.
The most widely method used for immunodiagnosis of fascioliasis is the ELISA. However, the antibody cross-reacts with other trematode antigens, including Schistosoma spp., giving false positive result (Hillyer 2005). Moreover, detection of Fasciola-specific antibodies does not discriminate between the previous and the recent infections. Thus, the use of specific antibodies to detect antigens secreted by the living flukes into their host’s body fluids may be a better approach not only to diagnose active infection but also to assess treatment efficacy and determine the effectiveness of future vaccines (Guobadia and Fagbemi 1996, 1997; Fagbemi et al. 1997). Therefore, the present study was conducted to evaluate the diagnostic capacity of one of Fasciola antigens, 14.5 kDa FABP, in diagnosis of human fascioliasis.
The FABP antigen was purified from the crude extracts by DEAE-sephadex A50-ion exchange chromatography and Sephacryl HR-100 gel filtration methods. FABP was appeared as a single band at 14.5 kDa by reducing SDS-PAGE. These yields were similarly reported by Raina et al. (2004) and Rabia et al. (2007). IgG polyclonal antibody (pAb) against 14.5 kDa FABP of F. gigantica were prepared and used for the detection of coproantigen in stool samples as well as the circulating Fasciola antigen in serum of infected human. Anti-FABP IgG was prepared by immunization of rabbits with FABP antigen. The purification procedures followed in this study were satisfactory for polyclonal IgG. Three purification steps were undertaken: ammonium sulfate precipitation, caprylic acid treatment, and ion exchange chromatography. The yield of anti-FABP IgG as a protein content by these methods was 1.1 mg/ml. This yield was reasonable in comparison with the yield of purified immunoglobulin from any biological fluid following similar purification procedures (Bride et al. 1995; Yang and Harrison 1996). The reactivity of the purified anti-FABP IgG was tested by indirect ELISA against FABP antigen. It was found that anti-FABP IgG pAb was highly sensitive, specific, and reliable for the detection of the FABP antigen.
Sandwich ELISA was employed to detect coproantigen in the stool of Fasciola-infected human using rabbit IgG raised against purified 14.5 kDa Fasciola antigen. Anti-FABP IgG successfully detected coproantigen in the stool samples with 96.43% sensitivity, 94.87% specificity, and 95.52% diagnostic efficacy. Such finding coincided with that obtained by El-Bassiouny et al. (2008) who found that using of monoclonal antibody against excretory-secretory (ES) antigens of F. gigantica for the detection of coproantigen in the stool gave 97% sensitivity and 97% specificity. Moreover, Rabia et al. (2007) reported that the sensitivity level of coproantigen detection assay in the stool samples of Fasciola-infected patients was 90% and the specificity was 94% using Fasciola purified tegumental antigen. In a field study, a survey screening for human fascioliasis using ELISA and coprological method in endemic location resulted in 95.5% sensitivity and 86.6% specificity (Espinoza et al. 2005). In addition, Moustafa et al. (1998) concluded that ELISA proved to be a rapid, easy, and sensitive test for diagnosing fascioliasis by detection of F. hepatica coproantigens earlier than routine stool examination. Fasciola coproantigens were detected in the stool of infected human patients several weeks before eggs being detectable in the stool (Youssef et al. 1991). Therefore, detection of coproantigens in stool is useful for early diagnosis of fascioliasis to avoid clinical complications of the disease.
In the current study, anti-FABP IgG was tested by sandwich ELISA to detect Fasciola antigen in sera collected from human patients. The sensitivity, specificity, and diagnostic efficacy of sandwich ELISA for detection of Fasciola antigen in serum samples was 94.47%, 84.62%, and 87.93%. These data were parallel to that achieved by Rabia et al. (2007) who recorded 91% sensitivity and 86.7% specificity when they used rabbit anti-FABP as antigen capture for detection of this Fasciola antigen by indirect ELISA in sera of infected animals. The sensitivity and specificity of serodiagnosis were slightly different depending on the antigen and the techniques used; 91% sensitivity and 92% specificity has been achieved by using anti-49.5 kDa Fasciola-specific ES fraction and sandwich ELISA for the detection of circulating antigens in fascioliasis patients (Espino and Finlay 1994; Espino et al. 1998). Sabry and Mohamed (2007) reported that the sandwich ELISA-detected antigen in sera of Fasciola-infected patients gave 92.4% sensitivity and 94.7% specificity when using rabbit anti-CP for antigen capture. They calculated 96.26% and 82.35% for positive and negative predictive values, respectively.
The results presented here indicated that the sandwich ELISA for detection of coproantigens is more sensitive and specific method for immunodiagnosis of fascioliasis. This result could be attributed to immune complex formation with host antibodies that tend to decrease the potential rate of circulating antigen. However, the levels of coproantigens were less affected by immune complex formation than circulating antigens (Mezo et al. 2007). Moreover, the use of serum samples has several disadvantages like antigenaemia that developed mainly during acute phase of infection (prepatent phase). After this period, antigen decrease and become undetectable over the course of infection (Espino et al. 1998). Therefore, coproantigens assay could be the most feasible procedure for the diagnosis of active, acute, and chronic infections, as serodiagnosis of fascioliasis is recommended only for early stages of infection.
Cross-reactivity with other parasites is a major problem in specificity of immunodiagnosis of fascioliasis, especially in countries endemic with schistosomiasis and fascioliasis (Carnevale et al. 2001; Rabia et al. 2007). A cross-reactivity between F. gigantica FABP antigens and other parasites antigens with varying degrees was evidenced in the present study, since Fasciola shares cross-reactive antigens with many parasites such as Schistosoma and Echinococcus (Espino et al. 1987; O’Neill et al. 1998). This cross-reaction may due to the presence of common antigenic lipoprotein components or presence of blood group substance in Fasciola cell membrane that play a role in the antigen sharing cross-reactivity (Ben-Ismail et al. 1982).
In conclusion, the present study clearly showed that the purified 14.5 kDa FABP obtained from F. gigantica adult worms could be introduced as a suitable candidate antigen for immunodiagnosis of human fascioliasis by using sandwich ELISA. The sensitivity and specificity of sandwich ELISA for the detection of Fasciola coproantigen in the stool were higher than those obtained by sandwich ELISA in the detection of circulating Fasciola antigen in serum.
References
Abdel-Rahman S, O’Reilly KL, Malone JB (1999) Biochemical characterization and localization of Fasciola hepatica 26–28 kDa diagnostic coproantigens. Parasite J Immunol 21:279–286
Anderson N, Luong TT, Vo GN, Bui LK, Smooker MP, Spithill WT (1999) The sensitivity and specificity of two methods for detecting Fasciola infections in cattle. Vet Parasitol 83:15–24
Ben-Ismail R, Mulet-Clamagirand C, Carme B, Gentilini M (1982) Biosynthesis of A, H, and Lewis blood group determinants in Fasciola hepatica. J Parasitol 68:402–407
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bride P, Jones P, Allen D, Donachie W, Huntley J, Mcconnell I, Hopkins J (1995) Analysis of the expression and secretion of isotypes of sheep B cell immunoglobulins with a panel of isotype-specific monoclonal antibodies. Res Vet Sci 59:189–194
Carnevale S, Rodriguez MI, Santillan G, Labbe JH, Cabrera MG, Bellegarde EJ, Velasquez JN, Trjoveic JJE, Guernera EA (2001) Immunodiagnosis of human fascioliosis by an enzyme-linked immunosorbent assay (ELISA) and a micro-ELISA. Clin Diagn Lab Immunol 8:174–177
El-Bassiouny A, Mohamed S, Salah F, Zoheiry M, Mansour W, Diab T, Rabia I, Safwat W, Demerdash Z (2008) Detection of Fasciola-specific coproantigens by sandwich ELISA using monoclonal antibodies. New Egypt J Med 36:115–120
Engels D, Nathimana S, De Vias SJ, Gryseels B (1997) Variation in weight of stool samples prepared by the Kato-Katz method and its implications. Trop Med Int Health 2:265–271
Engvall E, Perlman P (1971) Enzyme linked immunosorbent assay (ELISA). Quantitative assay of characterization immunoglobulin G. Immunochemistry 8:871–874
Espino AM, Finlay CM (1994) Sandwich enzyme–liked immunosorbentassay for detection of excretory-secretory antigens in human with fascioliasis. J Clin Microbiol 32:190–193
Espino AM, Dumenigo BE, Fernandez R, Finlay CM (1987) Immunodiagnosis of human fascioliasis be enzyme-linked immunosorbent assay using excretory-secretory product. AmJTrop Med Hyg 37:605–608
Espino AM, Marcet R, Finlay CM (1990) Detection of circulating excretory antigens in human fascioliasis by sandwich enzyme-linked immunosorbent assay. J Clin Microbiol 28:2637–2640
Espino AM, Millan JC, Finlay CM (1992) Detection of antibodies and circulating excretory secretory antigens for assessing cure of patients with fascioliasis. Trans R Soc Trop Med Hyg 86:649–654
Espino AM, Diaz A, Perez A, Finlay CM (1998) Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J Clin Microbiol 36:2723–2726
Espinoza JR, Timteo O, Herrera-Velit P (2005) Fas2-ELISA in the detection of human infection by Fasciola hepatica. J Helminthol 79:235–240
Esteban JG, Gonzalez C, Curtale F, Munoz-Antoli C, Valero MA, Bargues MD, El-Sayed M, El-Wakeel AA, Abdel-Wahab Y, Montresor A, Engels D, Savioli L, Mas-Coma S (2003) Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. AmJTrop Med Hyg 69:429–434
Estuningsih ES, Smooker PM, Wiedosari E, Widjajanti S, Vaiano A, Partotomo S, Spithill TW (1997) Evaluation of antigens of Fasciola gigantica as vaccine against tropical fascioliasis. Int J Parasitol 11:1419–1428
Fagbemi BO, Obarisiagbon IO, Mbuh JV (1995) Detection of circulating antigen in sera of Fasciola gigantica infected cattle with antibodies reactive with a Fasciola-specific 88-kDa antigen. Vet Parasitol 58:235–246
Fagbemi BO, Aderibigbe OA, Guobadia EE (1997) The use of monoclonal antibody for the immunodiagnosis of Fasciola gigantica infection in cattle. Vet Parasitol 69:230–240
García-Rodriguez JA, Martin Sánchez AM, Fernández Gorostarzu JM, García Luis EJ (1985) Fascioliasis in Spain: a review of the literature and personal observations. Eur J Epidemiol 1:121–126
Guobadia EE, Fagbemi BO (1996) Detection of circulating Fasciola gigantica antigen in experimental and natural infections of sheep with fascioliasis. Vet Parasitol 65:29–39
Guobadia EE, Fagbemi BO (1997) The isolation of F. gigantica-specific antigens and their use in the serodiagnosis of fascioliasis in sheep by the detection of circulating antigens. Vet Parasitol 68:269–282
Haseeb AN, EL-Shazly AM, Arafa MA, Morsy AT (2002) A review on fascioliasis in Egypt. J Egypt Soc Parasitol 32:317–354
Hillyer GV (1988) Fascioliasis and fasciolopsiasis. In: Baloes A, Hausler WJ, Ohashi M, Turano A (eds) Laboratory diagnosis of infectious diseases principles and practice. Springer-Verlag, Berlin, pp 856–862
Hillyer GV (1999) Immunodiagnosis of human and animal fascioliasis. In: Dalton JP (ed) Fascioliasis. CABI Publishing, New York, pp 435–448
Hillyer GV (2005) Fasciola antigens as vaccines against fascioliasis and schistosomiasis. J Helminthol 79:241–247
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685
Mas-Coma S (2004) Human fascioliasis. In: Cotruvo JA, Dufour A, Rees G et al (eds) Waterborne disease: identification, causes and control. WHO, IWA publishing, London, pp 305–318
Mas-Coma S (2005) Epidemiology of fascioliasis in human endemic areas. J Helminthol 79:207–216
Mas-Coma MS, Esteban JG, Bargues MD (1999) Epidemiology of human fascioliasis: a review and proposed new classification. Bull World Health Org 77:340–346
Mckinney MM, Parkinson A (1987) A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Meth 96:271–278
Mezo M, González-Warleta M, Ubeira FM (2007) The use of MM3 monoclonal antibodies for the early immunodiagnosis of ovine fascioliasis. J Parasitol 93:65–72
Moustafa NE, Hegab MH, Hassan MM (1998) Role of ELISA in early detection of Fasciola copro-antigens in experimentally infected animals. J Egypt Soc Parasitol 28:379–387
Nambi PA, Yadav SC, Raina OK, Sriveny D, Saini M (2005) Vaccination of buffaloes with Fasciola gigantica recombinant fatty acid binding protein. Parasitol Res 97:129–135
Nowotny A (1979) Basic exercises in immunochemistry. Springer Verlag, New York, pp 7–20
O’Neill SM, Parkinson M, Strauss W, Angles R, Dalton JP (1998) Immunodiagnosis of Fasciola hepatica infection (fascioliasis) in human population in the Bolivian Altiplano using purified cathepsin L cysteine proteinase. AmJTrop Med Hyg 58:417–423
Ockner RK (1990) Historic overviews of the studies on fatty acid binding proteins. Mol Cell Biochem 98:3–9
Rabia I, Salah F, Neamat M, Raafat A (2007) Evaluation of different antigens extracted from Fasciola gigantica for effective specific diagnosis of fascioliasis. New Egypt J Med 36:40–47
Raina OK, Sriveny D, Yadav SC (2004) Humoral immune response against Fasciola gigantica fatty acid binding protein. Vet Parasitol 124:65–72
Raina OK, Yadav SC, Sriveny D, Gupta SC (2006) Immuno-diagnosis of bubaline fasciolosis with Fasciola gigantica cathepsin-L and recombinant cathepsin L1-D proteases. Acta Trop 98:145–151
Ramajo V, Oleaga A, Casanueva P, Hillyer GV, Muro A (2001) Vaccination of sheep against Fasciola hepatica with homologous fatty acid binding proteins. Vet Parasitol 97:35–46
Sabry H, Mohamed S (2007) Diagnostic efficacy of anti-cysteine protease for detection of Fasciola antigen in serum and stool samples. New Egypt J Med 36:163–169
Sheehan D, FitzGerald RF (1996) Ion-exchange chromatography. Meth Mol Biol 59:145–150
Silvana C, Monica IR, Garciela S, Jorge HL, Marta GC, Enrique JB, Jorge NV, Jorge ET, Eduardo AG (2001) Immunodiagnosis of human fascioliasis by an enzyme-linked immunosorbent assay (ELISA) and micro ELISA. Clin Diagn Lab Immunol 8:174–177
Sirisriro A, Grams R, Vichasri-Grams S, Ardseungneon P, Pankao V, Meepool A, Chaithirayanon K, Viyanant V, Tan-Ariya P, Upatham ES, Sobhon P (2002) Production and characterization of a monoclonal antibody against recombinant fatty acid binding protein of Fasciola gigantica. Vet Parasitol 105:119–129
Snedecor GW, Cochran WG (1981) Statistical methods, 8th edn. the Lowa State University Press, Lowa, p 83
Spithill TW, Smooker PM, Copeman DB (1999) Fasciola gigantica: epidemiology, control, immunology and molecular biology. CAB International, Wallingford
Tijssen P, Kurstak P (1984) Highly efficient and simple methods for the preparation of peroxidase and active peroxidase-antibody conjugate for enzyme immunoassays. Anal Biochem 136:451–457
WHO (2004) Food–borne trematode infections in Asia. A joint WHO/FAO workshop. Rep Ser No RS/2002/GE/40 (VNT)
Yang YB, Harrison K (1996) Influence of column type and chromatographic conditions on the ion-exchange chromatography of immunoglobulins. J Chromatog A 743:171–180
Youssef FG, Mansour NS, Aziz AG (1991) Early diagnosis of human fascioliasis by the detection of copro-antigens using counterimmunoelectrophoresis. Trans R Soc Trop Med Hyg 85:383–384
Zane HD (2001) Laboratory safety and test quality assurance. In: Immunology: theoretical and practical concepts in laboratory medicine. Saunders WB, Philadelphia, pp 193–207
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allam, G., Bauomy, I.R., Hemyeda, Z.M. et al. Evaluation of a 14.5 kDa-Fasciola gigantica fatty acid binding protein as a diagnostic antigen for human fascioliasis. Parasitol Res 110, 1863–1871 (2012). https://doi.org/10.1007/s00436-011-2711-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2711-y