Abstract
Background
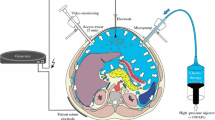
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel approach delivering intraperitoneal chemotherapy by means of a pressurized aerosol. This study was conducted to evaluate the distribution pattern of doxorubicin in the abdominal cavity after PIPAC in a postmortem swine model.
Methods
Doxorubicin was aerosolized through a Micropump© (MIP) into the peritoneal cavity of two swines at a pressure of 12 mm Hg CO2 and 32 °C. To measure the distribution of the drug, 9 different positions within the abdominal cavity were sampled. In-tissue doxorubicin penetration was evaluated using fluorescence microscopy on frozen thin sections.
Results
A maximum of drug penetration was observed in the area around the MIP. The penetration in the small intestine reached a depth of 349 ± 65 µm. Penetration depth in the right upper abdomen and left upper abdomen were 349 ± 65 and 140 µm ± 26 µm, respectively. Distant areas to the MIP showed variable penetration rates between 50 and 150 µm.
Conclusions
Doxorubicin reached all areas within the peritoneum. Highest penetration rates were measured in the area around the Micropump. Further studies are warranted to evaluate and optimize the distribution and penetration of cytotoxic agent into the tissue after PIPAC.



Similar content being viewed by others
Abbreviations
- CO2 :
-
Carbon dioxide
- CRS:
-
Cytoreductive surgery
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
- IAP:
-
Intra-abdominal pressure
- IPC:
-
Intraperitoneal chemotherapy
- PC:
-
Peritoneal carcinomatosis
- PCI:
-
Sugarbaker’s peritoneal cancer index
- PIPAC:
-
Pressurized intraperitoneal aerosol chemotherapy
- MIP® :
-
Micropump (Reger Medizintechnik, Rottweil, Germany)
References
Al-Quteimat OM, Al-Badaineh MA (2014) Intraperitoneal chemotherapy: rationale, applications, and limitations. J Oncol Pharm Pract 20(5):369–380
Chua TC, Esquivel J, Pelz JO, Morris DL (2013) Summary of current therapeutic options for peritoneal metastases from colorectal cancer. J Surg Oncol 107(6):566–573
Dedrick RL, Myers CE, Bungay PM, De Vita VT Jr. (1978) Pharmacokinetic rational for the peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 62(1):1–11
Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA (2016) Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis 18(4):364–371
Esquis P, Consolo D, Magnin G et al (2006) High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann Surg 244(1):106–112
Flessner MF (2005) The transport barrier in intraperitoneal therapy. Am J Physiol Renal Physiol 288(3):433–442
Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D (2008) Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol 15(1):339–344
Jacquet P, Sugarbaker PH (1996) Peritoneal-plasma barrier. Cancer Treat Res 82(1):53–63
Jacquet P, Stuart OA, Chang D, Sugarbaker PH (1996) Effects of intraabdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 7(5):596–603
Jain RK (1994) Barriers to drug delivery in solid tumors. Sci Am 271(1):58–65
Kakchekeeva T, Demtröder C, Herath NI et al (2016) In vivo feasibility of electrostatic precipitation as an adjunct to pressurized intraperitoneal aerosol chemotherapy (ePIPAC). Ann Surg Oncol (Epub ahead of print)
Khosrawipour V, Khosrawipour T, Falkenstein TA et al (2016a) Evaluating the effect of Micropump© position, internal pressure and doxorubicin dosage on efficacy of pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. Anticancer Res (Accepted for publication on 12 July 2016)
Khosrawipour V, Bellendorf A, Khosrawipour T et al (2016b) Irradiation does not increase the penetration depth of doxorubicin in normal tissue after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model. In Vivo 30(5):593–597
Khosrawipour V, Giger-Pabst U, Khosrawipour T et al (2016c) Effect of irradiation on tissue penetration depth of doxorubicin after pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex vivo model. J Cancer 7(8):910–914
Lotti M, Capponi MG, Piazzalunga D, Poisasina E, Pisano M, Manfredi R, Anssloni L (2016) Laparoscopic HIPEC: a bridge between open and closed-techniques. J Minim Access Surg 12(1):86–89
Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA (2016) Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg 20(2):367–373
Reymond MA, Solaß W (2014) Pressurized intraperitoneal aerosol chemotherapy—cancer under pressure, 1st edn. De Gruyter, Berlin
Sadeghi M, Arvieux C, Glehen O et al (2000) Peritoneal carcinomatosis from non-gynecological malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88(2):358–363
Solaß W, Hetzel A, Nadiradze G, Sagynaliev E, Reymond MA (2012a) Description of a novel approach for intraperitoneal drug delivery and the related device. Surg Endosc 26:1849–1855
Solass W, Herbette A, Schwarz T, Hetzel A, Sun JS, Dutreix M, Reymond MA (2012b) Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: proof of concept. Surg Endosc 26(3):847–852
Solass W, Kerb R, Mürdter T et al (2014) Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol 21(2):553–559
Sugarbaker PH (2003) Peritoneal carcinomatosis: Is cure an option? J Clin Oncol 21(5):762–764
Tempfer CB, Rezniczek GA, Ende P, Solass W, Reymond MA (2015) Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin in women with peritoneal carcinomatosis: a cohort study. Anticancer Res 35(12):6723–6729
Funding
This study was funded by institutional funds (Department of Surgery, Marien Hospital Herne, Ruhr University Bochum).
Authors’ contribution
Veria Khosrawipour contributed to study design, laboratory analysis, data acquisition and drafting of the manuscript; Tanja Khosrawipour contributed to laboratory analysis, data acquisition and drafting of the manuscript; Alexander Jens Peter Kern contributed to laboratory analysis, data acquisition and drafting of the manuscript; Aras Osma contributed to laboratory analysis and data acquisition; Burak Kabakci contributed to experimental and study design, laboratory analysis and data acquisition; David Diaz-Carballo contributed to supervision of the experiments and critical revision for important intellectual content of the manuscript; Eckart Förster contributed to study design, drafting and critical revision for important intellectual content of the manuscript; Jürgen Zieren contributed to supervision of the study, drafting and critical revision for important intellectual content of the manuscript; Khashayar Fakhrian contributed to study design, supervision of the study, data interpretation, drafting and critical revision for important intellectual content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human and animals rights
The swines were used and killed for a laparoscopic course at a local training center (Aesculap Akademie Bochum) prior to the experiment. This article does not contain any studies with human participants performed by any of the authors.
Ethical approval
An approval of the Local Board on Animal Care was obtained.
Rights and permissions
About this article
Cite this article
Khosrawipour, V., Khosrawipour, T., Kern, A.J.P. et al. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol 142, 2275–2280 (2016). https://doi.org/10.1007/s00432-016-2234-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2234-0