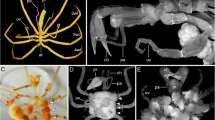
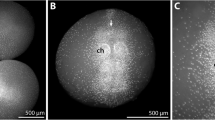
Abstract
Embryonic development of Pycnogonida (sea spiders) is poorly understood in comparison to other euarthropod lineages with well-established model organisms. However, given that pycnogonids potentially represent the sister group to chelicerates or even to all other euarthropods, their development might yield important data for the reconstruction of arthropod evolution. Using scanning electron microscopy, fluorescent nucleic staining and immunohistochemistry, the general course of embryonic morphogenesis in Pseudopallene sp. (Callipallenidae), a pycnogonid with prolonged embryonic development, is described. A staging system comprising ten stages is presented, which can be used in future studies addressing specific developmental processes. The initially slit-like stomodeum anlage forms at the anterior end of an eight-shaped germ band and predates proboscis outgrowth. The latter process is characterized by the protrusion of three cell populations that are subsequently involved in pharynx formation. In later stages, the proboscis assumes distally a horseshoe-like shape. At no time, a structure corresponding to the euarthropod labrum is detectable. Based on the complete lack of palpal and ovigeral embryonic limbs and the early differentiation of walking leg segments 1 and 2, the existence of an embryonized protonymphon stage during callipallenid development is rejected. The evolution of pycnogonid hatching stages, especially within Callipallenidae and Nymphonidae, is re-evaluated in the light of recent phylogenetic analyses. Specifically, the re-emergence of the ancestral protonymphon larva (including re-development of palpal and ovigeral larval limbs) and a possible re-appearance of adult palps in the nymphonid lineage are discussed. This challenges the perception of pycnogonid head appendage evolution as being driven by reduction events alone.







Similar content being viewed by others
References
Adlerz G (1888) Bidrag till pantopodernas morfologi och utvecklingshistoria. Bih Kungl Svenska Vet-Akad Handl 13:1–25
Anderson DT (1973) Embryology and phylogeny in annelids and arthropods. Pergamon Press, Oxford
Arabi J, Cruaud C, Couloux A, Hassanin A (2010) Studying sources of incongruence in arthropod molecular phylogenies: sea spiders (Pycnogonida) as a case study. C R Biol 333:438–453
Arango CP (2002) Morphological phylogenetics of the sea spiders (Arthropoda: Pycnogonida). Org Divers Evol 2:107–125
Arango CP (2003) Molecular approach to the phylogenetics of sea spiders (Arthropoda: Pycnogonida) using partial sequences of nuclear ribosomal DNA. Mol Phylogenet Evol 28:588–600
Arango CP, Wheeler WC (2007) Phylogeny of the sea spiders (Arthropoda, Pycnogonida) based on direct optimization of six loci and morphology. Cladistics 23:1–39
Bain BA (2003a) Postembryonic development in the pycnogonid Austropallene cornigera (Family Callipallenidae). Invertebr Reprod Dev 43:181–192
Bain BA (2003b) Larval types and a summary of postembryonic development within the pycnogonids. Invertebr Reprod Dev 43:193–222
Bamber RN (2007) A holistic re-interpretation of the phylogeny of the Pycnogonida Latreille, 1810 (Arthropoda). Zootaxa 1668:295–312
Bamber RN, El Nagar A (2011) Pycnobase: World Pycnogonida Database. Available online at http://www.marinespecies.org/pycnobase
Behrens W (1984) Larvenentwicklung und Metamorphose von Pycnogonum litorale (Chelicerata, Pantopoda). Zoomorphology 104:266–279
Bitsch J, Bitsch C (2007) The segmental organization of the head region in Chelicerata: a critical review of recent studies and hypotheses. Acta Zool 88:317–335
Bogomolova EV (2007) Larvae of three sea spider species of the genus Nymphon (Arthropoda: Pycnogonida) from the White Sea. Russian J Mar Biol 33:145–160
Bogomolova EV (2010) Nymphon macronyx (Arthropoda, Pycnogonida), another pycnogonid species with “lecytotrophic protonymphon” development. Zool Zhurnal 89:528–544
Bogomolova EV, Malakhov VV (2003) Larvae of sea spiders (Arthropoda, Pycnogonida) from the White Sea. Entomol Rev 83:222–236
Bogomolova EV, Malakhov VV (2004) Fine morphology of larvae of sea spiders (Arthropoda: Pycnogonida) from the White Sea. Zool Bespozvon 1:3–28
Bogomolova EV, Malakhov VV (2006) Lecithotrophic protonymphon is a special type of postembryonic development of sea spiders (Arthropoda, Pycnogonida). Dokl Biol Sci 409:328–331
Bourlat SJ, Nielsen C, Economou AD, Telford MJ (2008) Testing the new animal phylogeny: a phylum level molecular analysis of the animal kingdom. Mol Phylogenet Evol 49:23–31
Bouvier E-L (1923) Pycnogonides. Faune de France 7:1–70
Brenneis G, Ungerer P, Scholtz G (2008) The chelifores of sea spiders (Arthropoda, Pycnogonida) are the appendages of the deutocerebral segment. Evol Dev 10:717–724
Cano Sánchez E, López-González PJ (2010) Postembryonic development of Nymphon unguiculatum Hodgson 1915 (Pycnogonida, Nymphonidae) from the South Shetland Islands (Antarctica). Polar Biol 33:1205–1214
Cano E, López-González PJ (2009) Novel mode of postembryonic development in Ammothea genus (Pycnogonida: Ammotheidae) from Antarctic waters. Sci Mar 73:541–550
Child CA (1979) Shallow water Pycnogonida of the Isthmus of Panama and the coasts of Middle America. Smithson Contrib Zool 23:1–86
Child CA (1998) The marine fauna of New Zealand: Pycnogonida (sea spiders). NIWA Biodivers Mem 109:1–71
Chipman AD, Akam M (2008) The segmentation cascade in the centipede Strigamia maritima: involvement of the Notch pathway and pair-rule gene homologues. Dev Biol 319:160–169
Choe CP, Brown SJ (2009) Genetic regulation of engrailed and wingless in Tribolium segmentation and the evolution of pair-rule segmentation. Dev Biol 325:482–491
Dahms HU (2000) Phylogenetic implications of the crustacean nauplius. Hydrobiologia 417:91–99
Damen WGM (2007) Evolutionary conservation and divergence of the segmentation process in arthropods. Dev Dyn 236:1379–1391
Dogiel V (1913) Embryologische Studien an Pantopoden. Z Wiss Zool 107:575–741
Dohrn A (1881) Die Pantopoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel. Wilhelm Engelmann, Leipzig
Dunlop JA, Arango CP (2005) Pycnogonid affinities: a review. J Zool Syst Evol Res 43:8–21
Edgecombe GD, Wilson GDF, Colgan DJ, Gray MR, Cassis G (2000) Arthropod cladistics: combined analysis of histone H3 and U2 snRNA sequences and morphology. Cladistics 16:155–203
Eriksson BJ, Stollewerk A (2010) Expression patterns of neural genes in Euperipatoides kanangrensis suggest divergent evolution of onychophoran and euarthropod neurogenesis. Proc Natl Acad Sci USA 107:22576–22581
Eriksson BJ, Tait NN, Budd GE, Akam M (2009) The involvement of engrailed and wingless during segmentation in the onychophoran Euperipatoides kanangrensis (Peripatopsidae: Onychophora) (Reid 1996). Dev Genes Evol 219:249–264
Fahrenbach WH, Arango CP (2007) Microscopic Anatomy of Pycnogonida: II. Digestive System. III. Excretory System. J Morphol 268:917–935
Giribet G, Edgecombe GD, Wheeler WC (2001) Arthropod phylogeny based on eight molecular loci and morphology. Nature 413:157–161
Gnanamuthu CP (1950) Notes on the morphology and development of a pycnogonid, Propallene kempi (Calman), from Madras plankton. Proc Zool Soc Bengal 3:39–47
Hedgpeth JW (1947) On the evolutionary significance of the Pycnogonida. Smithson Misc Coll 106:1–53
Helfer H, Schlottke E (1935) Pantopoda. Dr. H. G. Bronns Klassen und Ordnungen des Tierreichs. Akademische Verlagsgesellschaft m.b.H., Leipzig
Henry LM (1953) The nervous system of the Pycnogonida. Microentomology 18:16–36
Hoek PPC (1881) Report on the Pycnogonida, dredged by H.M.S. Challenger during the years 1873–76. Zool Chall Exp 10:1–167
Hooper J (1980) Some aspects of the reproductive biology of Parapallene avida Stock (Pycnogonida: Callipallenidae) from Northern New South Wales. Aust Zool 30:473–483
Jager M, Murienne J, Clabaut C, Deutsch J, Le Guyader H, Manuel M (2006) Homology of arthropod anterior appendages revealed by Hox gene expression in a sea spider. Nature 441:506–508
Janssen R, Eriksson BJ, Budd GE, Akam M, Prpic NM (2010) Gene expression patterns in an onychophoran reveal that regionalization predates limb segmentation in pan-arthropods. Evol Dev 12:363–372
Koenemann S, Jenner RA, Hoenemann M, Stemme T, von Reumont BM (2010) Arthropod phylogeny revisited, with a focus on crustacean relationships. Arthropod Struct Dev 39:88–110
Lovely EC (2005) The life history of Phoxichilidium tubulariae (Pycnogonida: Phoxichilidiidae). Northeast Nat 12:77–92
Machner J, Scholtz G (2010) A scanning electron microscopy study of the embryonic development of Pycnogonum litorale (Arthropoda, Pycnogonida). J Morphol 271:1306–1318
Manuel M, Jager M, Murienne J, Clabaut C, Le Guyader H (2006) Hox genes in sea spiders (Pycnogonida) and the homology of arthropod head segments. Dev Genes Evol 216:481–491
Maxmen A, Browne WE, Martindale MQ, Giribet G (2005) Neuroanatomy of sea spiders implies an appendicular origin of the protocerebral segment. Nature 437:1144–1148
Meinert F (1899) Pycnogonida. The Danish Ingolf-Expedition. Bianco Luno (F. Dreyer), Copenhagen
Meisenheimer J (1902) Beiträge zur Entwicklungsgeschichte der Pantopoden. I. Die Entwicklung von Ammothea echinata Hodge bis zur Ausbildung der Larvenform. Z Wiss Zool 72:191–248
Meusemann K, von Reumont BM, Simon S, Roeding F, Strauss S, Kück P, Ebersberger I, Walzl M, Pass G, Breuers S, Achter V, von Haeseler A, Burmester T, Hadrys H, Wägele JW, Misof B (2010) A phylogenomic approach to resolve the arthropod tree of life. Mol Biol Evol 27:2451–2464
Miyazaki K (2002) On the shape of foregut lumen on sea spiders (Arthropoda: Pycnogonida). J Mar Biol Assoc UK 82:1037–1038
Morgan TH (1891) A contribution to the embryology and phylogeny of the pycnogonids. Stud Biol Lab Johns Hopkins Univ 5:1–76
Munilla T (1999) Evolución y filogenia de los picnogónidos. In: Melic A, de Haro JJ, Mendez M, Ribera I (eds) Evolución y filogenia de Arthropoda. Sociedad Entomológica Aragonesa, Zaragoza, pp 273–279
Nakamura K (1981) Post-embryonic development of a pycnogonid, Propallene longiceps. J Nat Hist 15:49–62
Nakamura K, Kano Y, Suzuki N, Namatame T, Kosaku A (2007) 18S rRNA phylogeny of sea spiders with emphasis on the position of Rhynchothoracidae. Mar Biol 153:213–223
Peel AD, Chipman AD, Akam M (2005) Arthropod segmentation: beyond the Drosophila paradigm. Nature Rev Genet 6:905–916
Prpic NM, Damen WGM (2009) Notch-mediated segmentation of the appendages is a molecular phylotypic trait of the arthropods. Dev Biol 326:262–271
Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW (2010) Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463:1079–1083
Rota-Stabelli O, Campbell L, Brinkmann H, Edgecombe GD, Longhorn SJ, Peterson KJ, Pisani D, Philippe H, Telford MJ (2011) A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc R Soc B 278:298–306
Sanchez S (1959) Le développement des Pycnogonides et leurs affinités avec les Arachnides. Arch Zool Exp Gén 98:1–102
Scholtz G (2000) Evolution of the nauplius stage in malacostracan crustaceans. J Zool Syst Evol Res 38:175–187
Scholtz G, Edgecombe GD (2006) The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev Genes Evol 216:395–415
Sekiguchi K, Nakamura K, Onuma S (1971) Egg-carrying habit and embryonic development in a pycnogonid, Propallene longiceps. Zool Mag 80:137–139
Stock JH (1994) Indo-west pacific Pycnogonida collected by some major oceanographic expeditions. Beaufortia 44:17–77
Stollewerk A (2008) Evolution of neurogenesis in arthropods. In: Minelli A, Fusco G (eds) Evolving pathways: key themes in evolutionary developmental biology. Cambridge University Press, Cambridge, pp 356–377
Ungerer P, Scholtz G (2008) Filling the gap between identified neuroblasts and neurons in crustaceans adds new support for Tetraconata. Proc R Soc B 275:369–376
Ungerer P, Scholtz G (2009) Cleavage and gastrulation in Pycnogonum litorale (Arthropoda, Pycnogonida): morphological support for the Ecdysozoa? Zoomorphology 128:263–274
Vilpoux K, Waloszek D (2003) Larval development and morphogenesis of the sea spider Pycnogonum litorale (Ström, 1762) and the tagmosis of the body of Pantopoda. Arthropod Struct Dev 32:349–383
von Reumont BM, Meusemann K, Szucsich NU, Dell’Ampio E, Gowri-Shankar V, Bartel D, Simon S, Letsch HO, Stocsits RR, Luan Y, Wägele JW, Pass G, Hadrys H, Misof B (2009) Can comprehensive background knowledge be incorporated into substitution models to improve phylogenetic analyses? A case study on major arthropod relationships. BMC Evol Biol 9:119
Walossek D, Müller KJ (1990) Upper Cambrian stem-lineage crustaceans and their bearing upon the monophyletic origin of Crustacea and the position of Agnostus. Lethaia 23:409–427
Westheide W, Rieger R (2007) Spezielle Zoologie. Teil 1: Einzeller und Wirbellose Tiere. Spektrum Akademischer Verlag, Heidelberg
Whiting MF, Bradler S, Maxwell T (2003) Loss and recovery of wings in stick insects. Nature 421:264–267
Whitington PM, Mayer G (2011) The origins of the arthropod nervous system: insights from the Onychophora. Arthropod Struct Dev 40:193–209
Winter G (1980) Beiträge zur Morphologie und Embryologie des vorderen Körperabschnitts (Cephalosoma) der Pantopoda Gerstaecker, 1863. I. Entstehung und Struktur des Zentralnervensystems. Z Zool Syst Evol-Forsch 18:27–61
Wirén E (1918) Zur Morphologie und Phylogenie der Pantopoden. Zool Bidr Uppsala 6:41–181
Wolff C, Scholtz G (2008) The clonal composition of biramous and uniramous arthropod limbs. Proc R Soc B 275:1023–1028
Zrzavý J, Hypsa V, Vlásková M (1997) Arthropod phylogeny: taxonomic congruence, total evidence and conditional combination approaches to morphological and molecular data sets. In: Fortey RA, Thomas RH (eds) Arthropod Relationships. Chapman and Hall, London, pp 97–107
Acknowledgments
Karen Gowlett-Holmes and Mick Baron are thanked for sharing their knowledge of Tasmanian dive sites and their invaluable help in specimen collection. The help of Paul Whitington, Roy Swain, Glenn Johnstone and Jonny Stark with the logistics of organizing the essential chemicals for processing embryos and larval stages is greatly appreciated. We are grateful to Wilfried Bleiss and Gabriele Drescher for the assistance with the scanning electron microscope. Petra Ungerer is thanked for critically reading parts of an earlier version of the manuscript. Collection of animals and their offspring was made possible by permits of the Tasmanian Department of Primary Industries, Parks, Water and Environment (permit nos. 6039 and 9255). Export of collected material was permitted by the Australian Department of the Environment, Water, Heritage and the Arts (permit nos. WT2008-4394 and WT2009-4260). GB was in part supported by the Studienstiftung des deutschen Volkes, and CPA was supported by Australian Biological Resources Study (ABRS, grant 204–61). The project was funded by the Deutsche Forschungsgemeinschaft (Scho 442/13-1)
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Roth
Electronic supplementary material
Below is the link to the electronic supplementary material.
Stomodeal cell immigration in ES 2 of Pseudopallene sp. Tubulin labeling (orange) with nucleic counterstain (green), Imaris volume (blend), same embryo as shown in Fig. 2c. The movie starts from anteroventral view, the embryo turns first in ventral then in lateral direction. Anteriorly in the germ band, a median tubulin-positive region is found where cells of the future stomodeum immigrate. The semicircular furrows of the chelifore anlagen are only indistinctly recognizable. An optical clipping plane along the sagittal axis of the embryo reveals the interiorly displaced nuclei of the immigrating stomodeal cells. (MPG 5076 kb)
Detail of the outgrowing proboscis in ES 4 of Pseudopallene sp. Tubulin labeling (orange) with nucleic counterstain (green), Imaris volume (blend). The movie starts from ventral view, the embryo rotates first laterally then dips backwards. The arrangement of the three cell populations creates the characteristic Y-shaped pharynx lumen. A distinct ring-like ridge of surrounding ectodermal cells starts to cover these cell populations (compare to Fig. 3g). At this stage, the medially touching chelifore anlagen are still positioned fully posterior to the proboscis. (MPG 7196 kb)
Detail of proboscis in ES 7 of Pseudopallene sp. Tubulin labeling (orange) with nucleic counterstain (green), Imaris volume (blend). The movie starts from ventral view, the embryo rotates first laterally then dips backwards. The developing mouth opening has a triangular shape. A distinct notch is found at the posterior margin of the proboscis, giving its distalmost portion a horseshoe-like appearance. The chelifore scapes that flank the proboscis laterally are damaged in this particular embryo. (MPG 6646 kb)
Rights and permissions
About this article
Cite this article
Brenneis, G., Arango, C.P. & Scholtz, G. Morphogenesis of Pseudopallene sp. (Pycnogonida, Callipallenidae) I: embryonic development. Dev Genes Evol 221, 309–328 (2011). https://doi.org/10.1007/s00427-011-0382-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-011-0382-4