Abstract
Schistosomiasis is a water-borne parasitic illness caused by neoophoran trematodes of the genus Schistosoma. Using classical histological techniques and whole-mount preparations, the present work describes the embryonic development of Schistosoma mansoni eggs in the murine host and compares it with eggs maintained under in vitro conditions. Two pre-embryonic stages occur inside the female worm: the prezygotic stage is characterized by the release of mature oocytes from the female ovary until its fertilization. The zygotic stage encompasses the migration of the zygote through the ootype, where the eggshell is formed, to the uterus. Fully formed eggs are laid still undeveloped, without having suffered any cleavage. In the outside environment, eight embryonic stages can be defined: stage 1 refers to early cleavages and the beginning of yolk fusion. Stage 2 represents late cleavage, with the formation of a stereoblastula and the onset of outer envelope differentiation. Stage 3 is defined by the elongation of the embryonic primordium and the onset of inner envelope formation. At stage 4, the first organ primordia arise. During stages 5 to 7, tissue and organ differentiation occurs (neural mass, epidermis, terebratorium, musculature, and miracidial glands). Stage 7 is characterized by the nuclear condensation of neurons of the central neural mass. Stage 8 refers to the fully formed larva, presenting muscular contraction, cilia, and flame-cell beating. This staging system was compared to a previous classification and could underlie further studies on egg histoproteomics (morphological localizome). The differentiation of embryonic structures and their probable roles in granulomatogenesis are discussed herein.








Similar content being viewed by others
References
Andrade ZA, Barka T (1962) Histochemical observations on experimental schistosomiasis of mouse. Am J Trop Med Hyg 11:12–16
Andrade ZA, Sadigursky M (1978) Immunofluorescence studies of schistosome structures which share determinants with circulating schistosome antigens. Trans R Soc Trop Med Hyg 72:316–317
Asahi H, Stadecker MJ (2003) Analysis of egg antigens inducing hepatic lesions in schistosome infection. Parasitol Int 52:361–367
Ashton PD, Harrop R, Shah B, Wilson RA (2001) The schistosome egg: development and secretions. Parasitology 122:329–338
Bahia D, Avelar LGA, Vigorosi F, Cioli D, Oliveira GC, Mortara RA (2006) The distribution of motor proteins in the muscles and flame cells of the Schistosoma mansoni miracidium and primary sporocyst. Parasitology 133:321–329
Bancroft JD, Stevens A (1996) Theory and practice of histological techniques, 4th edn. Churchill Livingstone, New York
Basch PF, Samuelson J (1990) Cell biology of schistosomes. I. Ultrastructure and transformations. In: Wyler DJ (ed) Modern parasite biology: cellular, immunological, and molecular aspects. Freeman, New York, pp 91–106
Baskic D, Popovic S, Ristic P, Arsenijevic NN (2006) Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol Int 30:924–932
Bobek L, Rekosh DM, van Keulen H, LoVerde PT (1986) Characterization of a female-specific cDNA derived from a developmentally regulated mRNA in the human blood fluke Schistosoma mansoni. Proc Natl Acad Sci U S A 83:5544–5548
Cardona A, Hartenstein V, Romero R (2005) The embryonic development of the triclad Schmidtea polychroa. Dev Genes Evol 215:109–131
Cardona A, Hartenstein V, Romero R (2006) Early embryogenesis of planaria: a cryptic larva feeding on maternal resources. Dev Genes Evol 216:667–681
Carson FL, Martin JH, Lynn JA (1973) Formalin fixation for electron microscopy: a re-evaluation. Am J Clin Pathol 59:365–373
Cass CL, Johnson JR, Califf LL, Xu T, Hernandez HJ, Stadecker MJ, Yates JR 3rd, Williams DL (2007) Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasitol 155:84–93
Chen LL, Rekosh DM, LoVerde PT (1992) Schistosoma mansoni p48 eggshell protein gene: characterization, developmentally regulated expression and comparison to the p14 eggshell protein gene. Mol Biochem Parasitol 52:39–52
Chernin E (1970) Behavioral responses of miracidia of Schistosoma mansoni and other trematodes to substances emitted by snails. J Parasitol 56:287–296
Chernin E (1974) Some host-finding attributes of Schistosoma mansoni miracidia. Am J Trop Med Hyg 23:320–327
Chitsulo L, Engels D, Montresor A, Savioli L (2000) The global status of schistosomiasis and its control. Acta Trop 77:41–51
Coelho MV (1957) Aspectos do desenvolvimento de formas larvárias de Schistosoma mansoni em Australorbis nigricans. Rev Bras Biol 17:325–337
Coelho PMZ, Andrade ZA, Borges C, Ribeiro F, Barbosa L (2008) Evolução do Schistosoma mansoni no Hospedeiro Intermediário. In: Carvalho ODS, Coelho PMZ, Lenzi HL (eds) Schistosoma mansoni e Esquistossomose: uma visão multidisciplinar. Fiocruz, Rio de Janeiro, pp 147–160
Conn DB, Swiderski Z (2008) A standardised terminology of the embryonic envelopes and associated developmental stages of tapeworms (Platyhelminthes: Cestoda). Folia Parasitol (Praha) 55:42–52
Cunha AS, Pellegrino J, Oliveira CA, Alvarenga RJ (1962) Observations on the oogram in guinea pigs and rabbits experimentally infected with Schistosoma mansoni. Rev Inst Med Trop Sao Paulo 4:242–248
Dalton JP, Day SR, Drew AC, Brindley PJ (1997) A method for the isolation of schistosome eggs and miracidia free of contaminating host tissues. Parasitology 115(Pt 1):29–32
de Araujo SC, de Mattos AC, Teixeira HF, Coelho PM, Nelson DL, de Oliveira MC (2007) Improvement of in vitro efficacy of a novel schistosomicidal drug by incorporation into nanoemulsions. Int J Pharm 337:307–315
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 7 Feb 2009
Dorsey CH, Cousin CE, Lewis FA, Stirewalt MA (2002) Ultrastructure of the Schistosoma mansoni cercaria. Micron 33:279–323
Dresden MH, Payne DC (1981) A sieving method for the collection of schistosome eggs from mouse intestines. J Parasitol 67:450–452
Ehlers U (1985) Das phylogenetische System der Plathelminthes. Fischer, Stuttgart
Eklu-Natey DT, Wuest J, Swiderski Z, Striebel HP, Huggel H (1985) Comparative scanning electron microscope (SEM) study of miracidia of four human schistosome species. Int J Parasitol 15:33–42
Erasmus DA (1975) The subcellular localization of labelled tyrosine in the vitelline cells of Schistosoma mansoni. Z Parasitenkd 46:75–81
Erasmus DA, Popiel I, Shaw JR (1982) A comparative study of the vitelline cell in Schistosoma mansoni, S. haematobium, S. japonicum and S. mattheei. Parasitology 84:283–287
Espin J (1941) La sustancia meplasmática en los nódulos producidos por Schistosoma mansoni. Rev Pol Caracas 10:73–90
Fitzpatrick JM, Johansen MV, Johnston DA, Dunne DW, Hoffmann KF (2004) Gender-associated gene expression in two related strains of Schistosoma japonicum. Mol Biochem Parasitol 136:191–209
Fitzpatrick JM, Hirai Y, Hirai H, Hoffmann KF (2007) Schistosome egg production is dependent upon the activities of two developmentally regulated tyrosinases. FASEB J 21:823–835
Freitas TC, Jung E, Pearce EJ (2007) TGF-beta signaling controls embryo development in the parasitic flatworm Schistosoma mansoni. PLoS Pathog 3:e52
Gobert GN, Chai M, McManus DP (2007) Biology of the schistosome lung-stage schistosomulum. Parasitology 134:453–460
Gonçalves T (2008) Correlação entre a difusão de material antigênico de ovo de Schistosoma mansoni com a expressão de moléculas de adesão de complexos juncionais em granulomas hepáticos murinos. Master's Dissertation, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro
Gonnert R (1955) Schistosomiasis-Studien, 1 Beitrage zur Anatomie und Histologie von Schistosoma mansoni. Z Tropenmed Parasitol 6:18–33
Gschwentner R, Ladurner P, Nimeth K, Rieger R (2001) Stem cells in a basal bilaterian. S-phase and mitotic cells in Convolutriloba longifissura (Acoela, Platyhelminthes). Cell Tissue Res 304:401–408
Haas W, Haberl B, Schmalfuss G, Khayyal MT (1994) Schistosoma haematobium cercarial host-finding and host-recognition differs from that of S. mansoni. J Parasitol 80:345–353
Haberl B, Kalbe M, Fuchs H, Ströbel M, Schmalfuss G, Haas W (1995) Schistosoma mansoni and S. haematobium: miracidial host-finding behaviour is stimulated by macromolecules. Int J Parasitol 25:551–560
Hartenstein V, Ehlers U (2000) The embryonic development of the rhabdocoel flatworm Mesostoma lingua (Abildgaard, 1789). Dev Genes Evol 210:399–415
Hartenstein V, Jones M (2003) The embryonic development of the body wall and nervous system of the cestode flatworm Hymenolepis diminuta. Cell Tissue Res 311:427–435
Hwang JS, Kobayashi C, Agata K, Ikeo K, Gojobori T (2004) Detection of apoptosis during planarian regeneration by the expression of apoptosis-related genes and TUNEL assay. Gene 333:15–25
Johnson KS, Taylor DW, Cordingley JS (1987) Possible eggshell protein gene from Schistosoma mansoni. Mol Biochem Parasitol 22:89–100
Jurberg AD, Oliveira AA, Lenzi HL, Coelho PM (2008a) A new miracidia hatching device for diagnosing schistosomiasis. Mem Inst Oswaldo Cruz 103:112–114
Jurberg AD, Pascarelli BM, Pelajo-Machado M, Maldonado A Jr, Mota EM, Lenzi HL (2008b) Trematode embryology: a new method for whole-egg analysis by confocal microscopy. Dev Genes Evol 218:267–271
Knobloch J, Kunz W, Grevelding CG (2006) Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni. Int J Parasitol 36:1261–1272
Koster B, Dargatz H, Schroder J, Hirzmann J, Haarmann C, Symmons P, Kunz W (1988) Identification and localisation of the products of a putative eggshell precursor gene in the vitellarium of Schistosoma mansoni. Mol Biochem Parasitol 31:183–198
Kusel JR, Oliveira FA, Todd M, Ronketti F, Lima SF, Mattos AC, Reis KT, Coelho PM, Thornhill JA, Ribeiro F (2006) The effects of drugs, ions, and poly-l-lysine on the excretory system of Schistosoma mansoni. Mem Inst Oswaldo Cruz 101(Suppl 1):293–298
Larsson LI, Bjerregaard B, Talts JF (2008) Cell fusions in mammals. Histochem Cell Biol 129:551–561
Lennert K (1978) Malignant lymphomas other than Hodgkin's disease. Springer, Berlin
Lenzi HL, Lenzi JA, Sobral AC (1987) Eosinophils favor the passage of eggs to the intestinal lumen in schistosomiasis. Br J Med Biol Res 20:433–435
Lenzi HL, Lenzi JA, Kerr IB, Antunes SL, Mota EM, Oliveira DN (1991) Extracellular matrix in parasitic and infectious diseases. Mem Inst Oswaldo Cruz 86:77–90
Lenzi HL, Jurberg AD, Coelho PMZ, Lenzi JA (2008) Migração e Desenvolvimento do Schistosoma mansoni no Hospedeiro Definitivo. In: Carvalho ODS, Coelho PMZ, Lenzi HL (eds) Schistosoma mansoni e Esquistossomose: uma visão multidisciplinar. Fiocruz, Rio de Janeiro, pp 85–145
Lewert RM, Lee CL (1954) Studies on the passage of helminth larvae through host tissues. I. Histochemical studies on the extracellular changes caused by penetrating larvae. II. Enzymatic activity of larvae in vitro and in vivo. J Infect Dis 95:13–51
Lewert RM, Para J, Ozcel MA (1970) Miracidial uptake of glucose in intact eggs of Schistosoma mansoni. J Parasitol 56:1250–1251
Lillie RD, Fullmer HM (1976) Histopathological technique and practical histochemistry. McGraw Hill, New York
Littlewood DTJ, Rohde K, Bray RA, Herniou EA (1999) Phylogeny of the Platyhelminthes and the evolution of parasitism. Biol J Linn Soc Lond 68:257–287
Machado-Silva JR, Pelajo-Machado M, Lenzi HL, Gomes DC (1998) Morphological study of adult male worms of Schistosoma mansoni Sambon, 1907 by confocal laser scanning microscopy. Mem Inst Oswaldo Cruz 93(Suppl 1):303–307
Maldonado JF, Acosta-Matienzo J (1947) Evolution del Schistosoma mansoni dentro de su hosped intermediário, el caracol Australorbis glabratus. P R J Public Health Trop Med 22:374–404
McLaren DJ (1980) Schistosoma mansoni: the parasite surface in relation to host immunity. Research Studies, New York
Michaels RM, Prata A (1968) Evolution and characteristics of Schistosoma mansoni eggs laid in vitro. J Parasitol 54:921–930
Michel A, Knobloch J, Kunz W (2003) P19: a female and tissue specifically expressed gene in Schistosoma mansoni, regulated by pairing with the male. Parasitology 127:519–524
Moore DV, Sandground JH (1956) The relative egg producing capacity of Schistosoma mansoni and Schistosoma japonicum. Am J Trop Med Hyg 5:831–840
Morris J, Nallur R, Ladurner P, Egger B, Rieger R, Hartenstein V (2004) The embryonic development of the flatworm Macrostomum sp. Dev Genes Evol 214:220–239
Neill PJ, Smith JH, Doughty BL, Kemp M (1988) The ultrastructure of the Schistosoma mansoni egg. Am J Trop Med Hyg 39:52–65
Neves RH, de Lamare Biolchini C, Machado-Silva JR, Carvalho JJ, Branquinho TB, Lenzi HL, Hulstijn M, Gomes DC (2005) A new description of the reproductive system of Schistosoma mansoni (Trematoda: Schistosomatidae) analyzed by confocal laser scanning microscopy. Parasitol Res 95:43–49
Nimeth K, Ladurner P, Gschwentner R, Salvenmoser W, Rieger R (2002) Cell renewal and apoptosis in Macrostomum sp. [Lignano]. Cell Biol Int 26:801–815
Oren-Suissa M, Podbilewicz B (2007) Cell fusion during development. Trends Cell Biol 17:537–546
Ottolina C (1957) El miracidio del Schistosoma mansoni: anatomía, citología, fisiología. Rev Sanid Asist Soc 22:1–412 discussion 413–420
Pan SC (1980) The fine structure of the miracidium of Schistosoma mansoni. J Invertebr Pathol 36:307–372
Pan SC (1996) Schistosoma mansoni: the ultrastructure of larval morphogenesis in Biomphalaria glabrata and of associated host–parasite interactions. Jpn J Med Sci Biol 49:129–149
Pellegrino J, Coelho PM (1978) Schistosoma mansoni: wandering capacity of a worm couple. J Parasitol 64:181–182
Pellegrino J, Faria J (1965) The oogram method for the screening of drugs in schistosomiasis mansoni. Am J Trop Med Hyg 14:363–369
Pellegrino J, Oliveira CA, Faria J, Cunha AS (1962) New approach to the screening of drugs in experimental schistosomiasis mansoni in mice. Am J Trop Med Hyg 11:201–215
Peralta JM (1986) Obtenção e caracterização de anticorpos monoclonais para antígenos de Schistosoma mansoni. Ph.D. Thesis, Instituto de Microbiologia, Universidade Federal do Rio de Janeiro-UFRJ, Rio de Janeiro
Podbilewicz B (2006) Cell fusion. WormBook, pp 1–32
Prata A (1957) Biópsia retal na esquistossomose mansoni — bases e aplicações no diagnóstico e tratamento. Serviço Nacional de Educação Sanitária-Ministério da Saúde, Rio de Janeiro
Ramachandra NB, Gates RD, Ladurner P, Jacobs DK, Hartenstein V (2002) Embryonic development in the primitive bilaterian Neochildia fusca: normal morphogenesis and isolation of POU genes Brn-1 and Brn-3. Dev Genes Evol 212:55–69
Reis MG, Kuhns J, Blanton R, Davis AH (1989) Localization and pattern of expression of a female specific mRNA in Schistosoma mansoni. Mol Biochem Parasitol 32:113–119
Saladin KS (1982) Schistosoma mansoni: cercarial responses to irradiance changes. J Parasitol 68:120–124
Sarvel AK, Kusel JR, Araujo N, Coelho P, Katz N (2006) Comparison between morphological and staining characteristics of live and dead eggs of Schistosoma mansoni. Mem Inst Oswaldo Cruz 101(Suppl 1):289–292
Schramm G, Gronow A, Knobloch J, Wippersteg V, Grevelding CG, Galle J, Fuller H, Stanley RG, Chiodini PL, Haas H, Doenhoff MJ (2006) IPSE/alpha-1: a major immunogenic component secreted from Schistosoma mansoni eggs. Mol Biochem Parasitol 147:9–19
Schussler P, Potters E, Winnen R, Bottke W, Kunz W (1995) An isoform of ferritin as a component of protein yolk platelets in Schistosoma mansoni. Mol Reprod Dev 41:325–330
Sorour F (1929) Contribution a l'etude des tumeurs irratitives bénignes et malignes produites par les Bilharzies. Ann Parasit 7:381–398
Stirewalt MA (1974) Schistosoma mansoni: cercaria to schistosomule. Adv Parasitol 12:115–182
Stjernholm RL, Warren KS (1974) Schistosoma mansoni: utilization of exogenous metabolites by eggs in vitro. Exp Parasitol 36:222–232
Swiderski Z (1994) Origin, differentiation and ultrastructure of egg envelopes surrounding the miracidia of Schistosoma mansoni. Acta Parasitol 39:64–72
Tyler S, Tyler MS (1997) Origin of the epidermis in parasitic platyhelminths. Int J Parasitol 27:715–738
Van de Vijver KK, Deelder AM, Jacobs W, Van Marck EA, Hokke CH (2006) LacdiNAc- and LacNAc-containing glycans induce granulomas in an in vivo model for schistosome egg-induced hepatic granuloma formation. Glycobiology 16:237–243
Vogel H (1942) Über Entwicklung, Lebensdauer und Tod der Eier vom Bilharzia japonica im Wirtsgewebe. Dtsch Tropenmed Ztsch 46:57–91
Warren KS (1978) The pathology, pathobiology and pathogenesis of schistosomiasis. Nature 273:609–612
Wells KE, Cordingley JS (1991) Schistosoma mansoni: eggshell formation is regulated by pH and calcium. Exp Parasitol 73:295–310
Williams DL, Asahi H, Botkin DJ, Stadecker MJ (2001) Schistosome infection stimulates host CD4(+) T helper cell and B-cell responses against a novel egg antigen, thioredoxin peroxidase. Infect Immun 69:1134–1141
Wilson RA (1987) Cercariae to liver worms: development and migration in the mammalian host. In: Rollinson D, Simpson AJG (eds) The biology of schistosomes: from genes to latrines. Academic, London, pp 115–146
World Health Organization (2006) Report of the Scientific Working Group meeting on Schistosomiasis. WHO, Geneva
Younossi-Hartenstein A, Hartenstein V (2000a) Comparative approach to developmental analysis: the case of the dalyellid flatworm, Gieysztoria superba. Int J Dev Biol 44:499–506
Younossi-Hartenstein A, Hartenstein V (2000b) The embryonic development of the polyclad flatworm Imogine mcgrathi. Dev Genes Evol 210:383–398
Younossi-Hartenstein A, Hartenstein V (2001) The embryonic development of the temnocephalid flatworms Craspedella pedum and Diceratocephala boschmai. Cell Tissue Res 304:295–310
Younossi-Hartenstein A, Ehlers U, Hartenstein V (2000) Embryonic development of the nervous system of the rhabdocoel flatworm Mesostoma lingua (Abilgaard, 1789). J Comp Neurol 416:461–474
Acknowledgments
The authors acknowledge the staff of the Laboratório de Patologia-IOC/Fiocruz and of the Laboratório de Esquistossomose-CPqRR/Fiocruz for technical assistance, Mr. Bruno Eschenazi from the Setor de Produção e Tratamento de Imagens-IOC/Fiocruz for the schematic drawing of S. mansoni egg embryonic development, Dr. Jane Arnt Lenzi from the Laboratório de Patologia-IOC/Fiocruz and Dr. John R Kusel from the Glasgow University for critical review of the manuscript, and Dr. Jennifer Rowland from the Laboratory of Patterning and Morphogenesis-Instituto Gulbenkian de Ciência for the English review. ADJ and TG received fellowships from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Brazil. TAC and ACA de M received fellowships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—Brazil. The work was supported by the Fundação Oswaldo Cruz (Fiocruz).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by D. A. Weisblat
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Interaction plots between each evaluated main effect. a Egg maturation stages at its source. b Egg source at its maturation stage. The nonparallel nature of lines connecting the mean area at levels of one main source put into a hierarchy by the levels of the other main source suggests interaction, as mentioned in the text. (GIF 62 kb)
Fig. S2
Model good fitting diagnostics. a Plot of residual quantiles against theoretical normal quantiles (Q–Q norm plot) shows a moderate tail and about 97% of data fitting the straight line, which suggests its normal distribution. b Box plot of padronized residuals shows a median value equal to null and a symmetric interquantile range (box), also suggesting a normal residual distribution. (GIF 47 kb)
Fig. S3
Distribution of the main effect levels of egg estimated areas during maturation. a Box plot of estimated areas of eggs for the source main effect levels, cultivated and isolated. Cultivated eggs are significant bigger than those eggs isolated from the murine host (P = 0.00017). b Box plot of estimated area of eggs for the maturation stage main effect levels, ordered in roman numerals(I to V). Eggs increase in size as embryos develop. (GIF 40 kb)
Fig. S4
Statistical analysis of egg growth. a, b Only the biologically relevant relationships were plotted, i.e., M-I:M-II, M-II:M-III, M-III:M-IV, and M-IV:M-V, where M is the maturation stage in roman numerals (according to Vogel and Prata's classification). a Simultaneous 95% confidence intervals for comparing estimated mean areas of cultivated eggs during growth under in vitro conditions (RPMI-1640 medium). b Simultaneous 95% confidence intervals for comparing estimated mean areas of isolated eggs during growth in the murine host. The overlapping of confidence intervals with the vertical dotted line at abscissa 0 indicates no pairwise difference between maturation stages at a level of confidence set as 0.05. Positive significant mean differences are higher than 0 for the lower intervals, while negative significant mean differences are lower than 0 for higher intervals. See details in the text. c Box plot of egg area during different stages of maturation (M) in regard to egg source (S). S1 refers to eggs obtained from the host and S2 refers to eggs maintained in culture. d Simultaneous 95% confidence intervals for comparing estimated mean areas of each matching stages for both sources (isolated and cultivated), i.e. M-I:M-I, M-II: M-II, M-III:M-III, M-IV:M-IV, and M-V:M-V. (GIF 92 kb)
Fig. S5
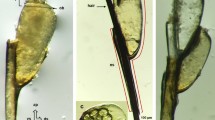
Oogram technique by bright field and confocal microscopies. a, b Bright field micrographs of compressed fragments of S. mansoni-infected mouse intestines. a Classical oogram. Three mature eggs are shown. b Compressed intestinal fragment stained with hydrochloric carmine analyzed by BM. It is difficult to identify the stages of maturation in each egg. c–e Confocal laser scanning microscope images of carmine-stained fragments of compressed intestines. c Sagittal section of two mature eggs (stage 8) and a third egg in an oblique view. nm, neural mass; np, neuropile; lg, lateral glands; gc, germinal cells. d Stage 2 egg, with the developing embryo (em). The stereoblastula and the yolk are visible. e Stage 3 egg, with an elongated embryo (em) and some yolk (yk). Scale bars 100 µm (GIF 69 kb)
Supplementary Table 1
Number of eggs counted per each Vogel and Prata's stage. The eggs were obtained from S. mansoni-infected mice and from culture. (DOC 29 kb)
Supplementary Table 2
Estimated mean areas (µm2) during S. mansoni egg growth in mice (isolated) and under in vitro conditions (cultivated). (DOC 169 kb)
Schistosoma mansoni embryo at stage 3 (MPG 3670 kb)
Schistosoma mansoni embryo at stage 6 (MPG 5014 kb)
Schistosoma mansoni embryo at stage 8 (mature miracidium) (MPG 3080 kb)
Rights and permissions
About this article
Cite this article
Jurberg, A.D., Gonçalves, T., Costa, T.A. et al. The embryonic development of Schistosoma mansoni eggs: proposal for a new staging system. Dev Genes Evol 219, 219–234 (2009). https://doi.org/10.1007/s00427-009-0285-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-009-0285-9