Abstract
Key message
High soil temperature during bulking and maturation of potatoes alters postharvest carbohydrate metabolism to attenuate genotypic resistance to cold-induced sweetening and accelerate loss of process quality.
Abstract
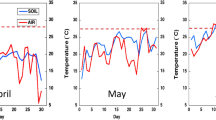
The effects of soil temperature during tuber development on physiological processes affecting retention of postharvest quality in low-temperature sweetening (LTS) resistant and susceptible potato cultivars were investigated. ‘Premier Russet’ (LTS resistant), AO02183-2 (LTS resistant) and ‘Ranger Russet’ (LTS susceptible) tubers were grown at 16 (ambient), 23 and 29 °C during bulking (111–164 DAP) and maturation (151–180 DAP). Bulking at 29 °C virtually eliminated yield despite vigorous vine growth. Tuber specific gravity decreased as soil temperature increased during bulking, but was not affected by temperature during maturation. Bulking at 23 °C and maturation at 29 °C induced higher reducing sugar levels in the proximal (basal) ends of tubers, resulting in non-uniform fry color at harvest, and abolished the LTS-resistant phenotype of ‘Premier Russet’ tubers. AO02183-2 tubers were more tolerant of heat for retention of LTS resistance. Higher bulking and maturation temperatures also accelerated LTS and loss of process quality of ‘Ranger Russet’ tubers, consistent with increased invertase and lower invertase inhibitor activities. During LTS, tuber respiration fell rapidly to a minimum as temperature decreased from 9 to 4 °C, followed by an increase to a maximum as tubers acclimated to 4 °C; respiration then declined over the remaining storage period. The magnitude of this cold-induced acclimation response correlated directly with the extent of buildup in sugars over the 24-day LTS period and thus reflected the effects of in-season heat stress on propensity of tubers to sweeten and lose process quality at 4 °C. While morphologically indistinguishable from control tubers, tubers grown at elevated temperature had different basal metabolic (respiration) rates at harvest and during cold acclimation, reduced dormancy during storage, greater increases in sucrose and reducing sugars and associated loss of process quality during LTS, and reduced ability to improve process quality through reconditioning. Breeding for retention of postharvest quality and LTS resistance should consider strategies for incorporating more robust tolerance to in-season heat stress.









Similar content being viewed by others
Abbreviations
- DAH:
-
Days after harvest
- DAP:
-
Days after planting
- DNS:
-
Dinitrosalicylic acid
- Fru:
-
Fructose
- Glc:
-
Glucose
- INV:
-
Acid invertase
- LTS:
-
Low-temperature sweetening
- NWPVD:
-
Northwest Potato Variety Development
- PM:
-
Physiological maturity
- PNW:
-
Pacific Northwest
- RAR:
-
Respiratory acclimation response
- REC:
-
Reconditioning period
- RS:
-
Reducing sugars
- SG:
-
Specific gravity
- SP:
-
Starch phosphorylase (L and H)
- SPS:
-
Sucrose-6-phosphate synthase
- TEA:
-
Triethanolamine
- WH:
-
Wound-healing
References
Amir J, Kahn V, Unterman M (1977) Respiration, ATP level, and sugar accumulation in potato tubers during storage at 4°C. Phytochemistry 16:1495–1498
Amrein TM, Bachmann S, Noti S, Beidermann M, Barbosa MF, Biedermann-Brem S, Grob K, Keiser a, Realini P, Escher F, Amado R (2003) Potential acrylamide formation, sugars, and free asparagine in potatoes: a comparison of cultivars and farming systems. J Agric Food Chem 51:5556–5560
Bergmeyer HU, Bernt E (1974) Sucrose. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1176–1179
Bergmeyer HU, Bernt E, Schmidt F, Stork H (1974) Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1196–1201
Bernt E, Bergmeyer HU (1974) d-Fructose. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1304–1307
Bhaskar PB, Wu L, Busse JS, Whitty BR, Hamernik AJ, Jansky SH, Buell CR, Bethke PC, Jiang J (2010) Suppression of the vacuolar invertase gene prevents cold-induced sweetening in potato. Plant Physiol 154:939–948
Blauer JM, Knowles LO, Knowles NR (2013a) Evidence that respiration is the pacemaker of physiological aging in seed potatoes (Solanum tuberosum L.). J Plant Growth Regul 32:708–720
Blauer JM, Kumar GNM, Knowles LO, Dhingra A, Knowles NR (2013b) Changes in ascorbate and associated gene expression during development and storage of potato tubers (Solanum tuberosum L.). Post Biol Tech 78:76–91
Blenkinsop RW, Yada RY, Marangoni AG (2004) Metabolic control of low-temperature sweetening in potato tubers during postharvest storage. Hort Rev 30:317–354
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantity of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brummell DA, Chen RKY, Harris JC, Zhang H, Hamiaux C, Kralicek AV, McKenzie MJ (2011) Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J Exp Bot 62:3519–3534
Claassen PAM, Budde MAW, van Calker MH (1993) Increase in phosphorylase activity during cold-induced sugar accumulation in potato tubers. Potato Res 36:205–217
Dam JV, Kooman PL, Struik PC (1996) Effects of temperature and photoperiod on early growth and final number of tubers in potatoes (Solanum tuberosum L.). Potato Res. 39:51–62
Davies HV, Viola R (1992) Regulation of sugar accumulation in stored potato tubers. Postharv News Info 3:97–100
Driskill EP, Knowles LO, Knowles NR (2007) Temperature-induced changes in potato processing quality during storage are modulated by tuber maturity. Amer J Potato Res 84:367–383
Epstein E (1966) Effects of soil temperature at different growth stages on growth and development of potato plants. Agron J 58:169–171
Fettke J, Albrecht T, Hejazi M, Mahlow S, Nakamura Y, Steup M (2010) Glucose-1-phosphate is efficiently taken up by potato (Solanum tuberosum L.) tuber parenchyma cells and converted to reserve starch granules. New Phytol 185:663–675
Fettke J, Leifels L, Brust H, Herbst K, Steup M (2012) Two carbon fluxes to reserve starch in potato (Solanum tuberosum L.) tuber cells are closely interconnected but differently modulated by temperature. J Exp Bot 63:3011–3029
Geigenberger P, Geiger M, Stitt M (1998) High-temperature perturbation of starch synthesis is attributed to the inhibition of ADP-Glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. Plant Physiol 117:1307–1316
Gould WA (1999) Potato production, processing, and technology. CTI Publications, Inc., Timonium, p 61
Greiner S, Rausch T, Sonnewald U, Herbers K (1999) Ectopic expression of a tobacco invertase inhibitor homolog prevents cold-induced sweetening of potato tubers. Nat Biotechnol 17:708–711
Hill LM, Reimholz R, Schroder R, Nielson TH, Stitt M (1996) The onset of sucrose accumulation in cold-stored potato tubers is caused by an increased rate of sucrose synthesis and coincides with low levels of hexose-phosphates, an activation of sucrose phosphate synthase and the appearance of a new form of amylase. Plant Cell Environ 19:1223–1237
Hiller LK, Koller DC, Thornton RE (1985) Physiological disorders of potato tubers. In: Li PH (ed) Potato physiology. Academic, New York, pp 389–443
Ingram KT, McCloud DE (1984) Simulation of potato crop growth and development. Crop Sci 24:21–27
Iritani WM, Weller LD (1980) Sugar development in potatoes. Coop Ext. Bull. #0717, Washington State University, Pullman, WA
Isherwood FA (1973) Starch-sugar interconversion in Solanum tuberosum. Phytochemistry 12:2579–2591
Isherwood FA (1976) Mechanism of sugar-starch interconversion in Solanum tuberosum. Phytochemistry 15:33–41
Isherwood FA, Burton WG (1975) The effect of senescence, handling, sprouting and chemical sprout suppression upon the respiratory quotient of stored potato tubers. Potato Res 18:98–104
Kinkaid DC, Westermann DT, Trout TJ (1993) Irrigation and soil temperature effects on Russet Burbank quality. Am Potato J 70:711–723
Kleinkopf GE, Westermann DT, Wille MJ (1988) Soil temperature effects on sugar end development. Proc Univ Idaho Winter Comm Schools 20:180–181
Knowles NR, Knowles LO (2006) Manipulating stem number, tuber set, and yield relationships for northern- and southern-grown potato seed lots. Crop Sci 46:284–296
Knowles NR, Plissey ES (2007) Maintaining tuber health during harvest, storage, and post-storage handling. In: Johnson DA (ed) Potato health management, 2 edn. APS, St Paul, pp 79–99
Knowles NR, Pavek MJ, Knowles LO, Holden Z (2008) Developmental profiles and postharvest behavior of long-season processing cultivars. In: Proceedings of the 47th Annual Washington State Potato Conference, 5–7 Feb, Moses Lake, WA, pp 45–46
Knowles NR, Driskill EP, Knowles LO (2009) Sweetening responses of potato tubers of different maturity to conventional and non-conventional storage temperature regimes. Post Biol Tech 52:49–61
Knowles NR, Knowles LO, Pavek MJ (2013) In-season management and post-harvest quality lessons learned from Alpine Russet, Sage Russet and other cultivars. In: Proceedings of the 47th Annual Washington State Potato Conference, 29–31 Jan, Moses Lake, WA, pp 34–47
Kooman PL, Fahem M, Tegera P, Haverkort AJ (1996) Effects of climate on different potato genotypes 2. Dry matter allocation and duration of the growth cycle. Eur J Agron 5:207–217
Krause KP, Hill L, Reimholz R, Hamborg Nielson T, Sonnewald U, Stitt M (1998) Sucrose metabolism in cold-stored potato tubers with decreased expression of sucrose phosphate synthase. Plant Cell Environ 21:285–299
Krauss A, Marshner H (1984) Growth rate and sugar metabolism of potato tubers exposed to high temperature. Potato Res 27:297–303
Kumar GNM, Iyer S, Knowles NR (2007) Extraction of RNA from fresh, frozen and lyophilized tuber and root tissues. J Agric Food Chem 55:1674–1678
Lafta AM, Lorenzen JH (1995) Effects of high temperature on plant growth and carbohydrate metabolism in potatoes. Plant Physiol 109:637–643
Lenfesty CM (1967) Soil Survey: Adams County, Washington. United State Soil Conservation Service, Washington DC
Liu X, Lin Y, Liu J, Song B, Ou Y, Zhang H, Li M, Xie C (2013) StInvInh2 as an inhibitor of StvacINV1 regulates cold-induced sweetening of potato tubers by specifically capping vacuolar invertase activity. Plant Biotech J 11:640–647
Manrique LA, Bartholomew DP (1991) Growth and yield performance of potato grown at three elevations in Hawaii. II. Dry matter production and efficiency of partitioning. Crop Sci 31:367–372
McKenzie MJ, Chen RKY, Harris JC, Ashworth MJ, Brummell DA (2013) Post-translational regulation of acid invertase activity by vacuolar invertase affects resistance to cold-induced sweetening of potato tubers. Plant Cell Environ 36:176–185
National Agricultural Statistics Service (USDA) (2012) Potatoes 2011 summary: September 2012. ISSN: 1949–1514
Novy RG, Whitworth JL, Stark JC, Love SL, Corsini DL, Pavek JJ, Vales MI, James SR, Hane DC, Shock CC, Charlton BA, Brown CR, Knowles NR, Pavek MJ, Brandt TL, Olsen N (2008) Premier Russet: a dual-purpose, potato cultivar with significant resistance to low-temperature sweetening during long-term storage. Am J Pot Res 85:198–209
Pavek MJ, Knowles NR (2004–2012) Potato cultivar yield and postharvest quality evaluations. Washington State University Special Report. http://www.potatoes.wsu.edu
Pritchard MK, Adam LR (1992) Preconditioning and storage of chemically immature Russet Burbank and Shepody potatoes. Am Potato J 69:805–815
Rathore RS, Garg N, Garg S, Kumar A (2009) Starch phosphorylase: role in starch metabolism and biotechnological applications. Crit Rev Biotech 29:214–224
Reynolds MP, Ewing EE (1989) Effects of high air temperature stress on growth and tuberization in Solanum tuberosum. Ann Bot 64:241–247
Sarquis JI, Gonzalez H, Bernal-Lugo I (1996) Response of two potato clones (S. tuberosum L.) to contrasting temperature regimes in the field. Am Potato Res 73:285–300
Schippers PA (1977) The rate of respiration of potato tubers during storage 1. Review of literature. Potato Res 20:173–188
Shallenberger RS, Smith O, Treadway RH (1959) Role of the sugars in browning reaction in potato chips. J Agric Food Chem 7:274–277
Snyder RG, Ewing EE (1989) Interactive effects of temperature, photoperiod, and cultivar on tuberization of potato cuttings. HortScience 24:336–338
Sowokinos JR (1990) Stress-induced alterations in carbohydrate metabolism. In: Vayda ME, Park WD (eds) The molecular and cell biology of the potato. CAB Int., Wallingford, pp 137–158
Sowokinos JR (2001) Biochemical and molecular control of cold-induced sweetening in potatoes. Am J Potato Res 78:221–236
Sowokinos JR, Shock CC, Stieber TD, Eldredge EP (2000) Compositional and enzymatic changes associated with the sugar-end defect in Russet Burbank potatoes. Am J Potato Res 77:47–56
Steup M (1990) Starch degrading enzymes. In: Lea PJ (ed) Methods in plant biochemistry, vol 3. Elsevier, Amsterdam, pp 103–128
Struik PC, van der Putten PEL, Caldiz DO, Scholte K (2006) Response of stored potato seed tubers from contrasting cultivars to accumulated degree-days. Crop Sci 46:1156–1168
Temmerman LD, Wolf J, Colls J, Bindi M, Fangmeier A, Finnan J, Ojanpera K, Pleijel H (2002) Effects of climatic conditions on tuber yield (Solanum tuberosum L.) in the European ‘CHIP’ experiments. Eur J Agron 17:243–255
Thompson AL, Love SL, Sowokinos JR, Thornton MK, Shock CC (2008) Review of the sugar end disorder in potato (Solanum tuberosum L.). Am J Potato Res 85:375–386
Timlin D, Lutfor RSM, Baker J, Reddy VR, Fleisher D, Quebedeaux B (2006) Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron J 98:1195–1203
Viola R, Davies HV, Chudek AR (1991) Pathways of starch and sucrose biosynthesis in developing tubers of potato (Solanum tuberosum L.) and seeds of faba bean (Vicia faba L.). Elucidation by 13C NMR spectroscopy. Planta 183:202–208
Yamaguchi M, Timm H, Spurr AR (1964) Effects of soil temperature on growth and nutrition of potato plants and tuberization, composition, and periderm structure of tubers. Proc Am Soc Hortic Sci 84:412–423
Zommick DH, Kumar GNM, Knowles LO, Knowles NR (2013) Translucent tissue defect in potato (Solanum tuberosum L.) tubers is associated with oxidative stress accompanying an accelerated aging phenotype. Planta 238:1125–1145
Zrenner R, Schuler K, Sonnewald U (1996) Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198:246–252
Acknowledgments
Financial support provided by the USDA ARS, Washington State Potato Commission and WSU Agricultural Research Center to N.R.K. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zommick, D.H., Knowles, L.O., Pavek, M.J. et al. In-season heat stress compromises postharvest quality and low-temperature sweetening resistance in potato (Solanum tuberosum L.). Planta 239, 1243–1263 (2014). https://doi.org/10.1007/s00425-014-2048-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-014-2048-8