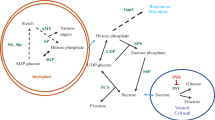
Abstract
Main conclusion
Tolerance to heat stress for retention of low-temperature sweetening-resistant phenotype in potato is conferred by insensitivity of acid invertase activity to cold induction.
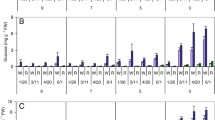
Heat stress exacerbated cold sweetening (buildup of reducing sugars) of the LTS (low-temperature sweetening)-susceptible potato (Solanum tuberosum L.) cultivars, Ranger Russet and Russet Burbank, and completely abolished the resistance to cold sweetening in the LTS-resistant cultivars/clones, Sage Russet, GemStar Russet, POR06V12-3 and A02138-2. Payette Russet and EGA09702-2, however, demonstrated considerable tolerance to heat stress for retention of their LTS-resistant phenotype. Heat-primed Payette Russet and EGA09702-2 tubers accumulated fourfold more sucrose when subsequently stored at 4 °C, while reducing sugar concentrations also increased marginally but remained low relative to the non-heat-tolerant LTS-resistant clones, resulting in light-colored fries. By contrast, sucrose concentrations in heat-primed tubers of the non-heat-tolerant clones remained unchanged during LTS, but reducing sugars increased fivefold, resulting in darkening of processed fries. Acid invertase activity increased in the LTS-susceptible and non-heat-tolerant LTS-resistant cultivars/clones during cold storage. However, Payette Russet tubers maintained very low invertase activity regardless of heat stress and cold storage treatments, as was the case for Innate® Russet Burbank (W8) tubers, where silenced invertase conferred robust tolerance to heat stress for retention of LTS-resistant phenotype. Importantly, heat-stressed tubers of Payette Russet, EGA09702-2 and Innate® Russet Burbank (W8) demonstrated similar low reducing sugar and high sucrose-accumulating phenotypes when stored at 4 °C. Tolerance to heat stress for retention of LTS-resistant phenotype in Payette Russet and likely its maternal parent, EGA09702-2, is, therefore, conferred by the ability to maintain low invertase activity during cold storage of heat-stressed tubers.










Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- CS:
-
Cold stress
- DAH:
-
Days after harvest
- DAP:
-
Days after planting
- Fru:
-
Fructose
- Glc:
-
Glucose
- HS:
-
Heat stress
- Inh1:
-
Apoplastic invertase inhibitor 1
- Inh2:
-
Vacuolar invertase inhibitor 2 β
- LTS:
-
Low-temperature sweetening
- Mkt:
-
Marketable yield (total yield minus culls)
- PHHS:
-
Postharvest heat stress
- WH:
-
Wound healing
References
Bergmeyer HU, Bernt E (1974) Sucrose. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1176–1179
Bergmeyer HU, Bernt E, Schmidt F, Stork H (1974) Determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1196–1201
Bernt E, Bergmeyer HU (1974) d-Fructose. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie-Academic Press, New York, pp 1304–1307
Bethke PC (2013) Postharvest storage and physiology. In: Navarre R, Pavek MJ (eds) The potato: botany, production, and uses. CABI, Boston, pp 255–271
Blauer JM, Knowles LO, Knowles NR (2013) Evidence that tuber respiration is the pacemaker of physiological aging in seed potatoes (Solanum tuberosum L.). J Plant Growth Regul 32:708–720
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantity of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brummell DA, Chen RKY, Harris JC, Zhang H, Hamiaux C, Kralicek AV, McKenzie MJ (2011) Induction of vacuolar invertase inhibitor mRNA in potato tubers contributes to cold-induced sweetening resistance and includes spliced hybrid mRNA variants. J Exp Bot 62:3519–3534
Burton WG (1966) The potato, 2nd edn. Wageningen, Veenman, p 382
Burton WG (1978) Post-harvest behaviour and storage of potatoes. In: Crocker TH (ed) Applied biology III. Academic Press, London, pp 86–228
Clark P, Habig J, Ye J, Collinge S (2014). Petition for determination of nonregulated status for Innate™ potatoes with late blight resistance, low acrylamide potential, reduced black spot, and lowered reducing sugars: Russet Burbank event W8. J.R. Simplot Company Petition JRS01 (USDA Petition 14-093-01p)
Driskill EP, Knowles LO, Knowles NR (2007) Temperature induced changes in potato processing quality during storage are modulated by tuber maturity. Am J Potato Res 84:367–383
Geigenberger P, Geiger M, Stitt M (1998) High-temperature perturbation of starch synthesis is attributed to the inhibition of ADP-Glucose pyrophosphorylase by decreased levels of glycerate-3-phosphate in growing potato tubers. Plant Physiol 117:1307–1316
Gould WA (1999) Potato production, processing, and technology. CTI Publications, Timonium, p 61
Herman DJ, Knowles LO, Knowles NR (2016) Low oxygen storage modulates invertase activity to attenuate cold-induced sweetening and loss of process quality in potato (Solanum tuberosum L.). Postharvest Biol Tech 121:106–117
Hill LM, Reimholz R, Schroder R, Nielson TH, Stitt M (1996) The onset of sucrose accumulation in cold-stored potato tubers is caused by an increased rate of sucrose synthesis and coincides with low levels of hexose-phosphates, an activation of sucrose phosphate synthase and the appearance of a new form of amylase. Plant Cell Environ 19:1223–1237
International Agency for Research on Cancer (1994) Some industrial chemicals: summary of data reported and evaluation. IARC Monographs on the Evaluation of Carcinogenic Risks to Human 60. http://monographs.iarc.fr/ENG/Monographs/vol60/mono60-16.pdf. Accessed 14 April 2016
Isherwood FA (1976) Mechanism of sugar-starch interconversion in Solanum tuberosum. Phytochemistry 15:33–41
Knowles NR, Pavek MJ (2007) WSU potato cultivar yield and postharvest quality evaluations for 2006. Washington State University Special Report
Knowles NR, Pavek MJ (2013) WSU potato cultivar yield and postharvest quality evaluations for 2012. Washington State University Special Report
Knowles NR, Driskill EP, Knowles LO (2009) Sweetening responses of potato tubers of different maturity to conventional and non-conventional storage regimes. Postharvest Biol Tech 52:49–61
Krauss A, Marshner H (1984) Growth rate and sugar metabolism of potato tubers exposed to high temperature. Potato Res 27:297–303
Kumar D, Singh BP, Kumar P (2004) An overview of the factors affecting sugar content of potatoes. Ann Appl Biol 145:247–256
Kumar GNM, Knowles LO, Knowles NR (2015) Zebra chip disease decreases tuber (Solanum tuberosum L.) protein content by attenuating protease inhibitor levels and increasing protease levels. Planta 242:1153–1166
Lafta AM, Lorenzen JH (1995) Effects of high temperature on plant growth and carbohydrate metabolism in potatoes. Plant Physiol 109:637–643
Lenfesty CM (1967) Soil survey: Adams County, Washington. Washington D.C.
Lin Y, Liu T, Liu X, Ou Y, Zhang H, Li M, Sonnewald U, Song B, Xie C (2015) Subtle regulation of potato acid invertase activity by a protein complex of invertase, invertase inhibitor, and SUCROSE NONFERMENTING1-RELATED PROTEIN KINASE. Plant Physiol 168:1807–1819
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCT method. Methods 25:402–408
Love SL, Novy RG, Whitworth J, Corsini DL, Pavek JJ, Mosley AR, Pavek MJ, Knowles NR, Brown CR, James SR, Hare DC, Miller JC (2006) GemStar Russet: a potato variety with high yield, good culinary quality, excellent fresh market appearance, and resistance to common scab. Am J Potato Res 83:171–180
Malone JG, Mittova V, Ratcliffe RG, Kruger NJ (2006) The response of carbohydrate metabolism in potato tubers to low temperature. Plant Cell Physiol 47:1309–1322
McKenzie MJ, Sowokinos JR, Shea IM, Gupta SK, Lindlauf RR, Anderson JAD (2005) Investigations on the role of acid invertase and UDP-glucose pyrophosphorylase in potato clones with varying resistance to cold-induced sweetening. Am J Potato Res 82:231–239
McKenzie MJ, Chen RKY, Harris JC, Ashworth MJ, Brummell DA (2013) Post-translational regulation of acid invertase activity by vacuolar invertase affects resistance to cold-induced sweetening of potato tubers. Plant Cell Environ 36:176–185
Midmore DJ (1992) Potato production in the tropics. In: Harris P (ed) The potato crop: the scientific basis for improvement, 2nd edn. Chapman and Hall, London, pp 728–793
Midmore DJ, Prange RK (1992) Growth responses of two Solanum species to contrasting temperatures and irradiance levels: relation to photosynthesis, dark respiration and chlorophyll fluorescence. Ann Bot 69:13–20
Midmore DJ, Roca J (1992) Influence of production and storage conditions on subsequent growth and tuber yield of potato (Solanum spp.) in the hot tropics. J Agric Sci 119:45–58
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2:405–410
Mohabir G, John P (1988) Effect of temperature on starch synthesis in potato tuber tissue and in amyloplasts. Plant Physiol 88:1222–1228
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419:448–449
Nicot N, Hausman J, Hoffman L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Nielsen TH, Deiting U, Stitt M (1997) α-amylase in potato tubers is induced by storage at low temperature. Plant Physiol 113:503–510
Novy RG, Whitworth JL, Stark JC, Love SL, Corsini DL, Pavek JJ, Vales MI, James SR, Hane DC, Shock CC, Charlton BA, Brown CR, Knowles NR, Pavek MJ, Brandt TL, Olsen N (2008) Premier Russet: a dual-purpose potato cultivar with significant resistance to low temperature sweetening during long-term storage. Am J Potato Res 85:198–209
Novy RG, Whitworth JL, Stark JC, Love SL, Corsini DL, Pavek JJ, Vales IM, James SR, Hane DC, Shock CC, Charlton BA, Brown CR, Knowles NR, Pavek MJ, Brandt TL, Gupta S, Olsen N (2010) Clearwater Russet: a dual-purpose potato cultivar with cold sweetening resistance, high protein content, and a low incidence of external defects and sugar ends. Am J Potato Res 87:458–471
Novy RG, Whitworth JL, Stark JC, Schneider B, Knowles NR, Pavek MJ, Knowles LO, Charlton BA, Sathuvalli V, Yilma S, Brown CR, Thornton M, Brandt TL, Olsen N (2016) Payette Russet: a dual-purpose cultivar with cold-sweetening resistance, low acrylamide formation, and resistance to late blight and potato virus Y. Am J Potato Res (in press)
Ou Y, Song B, Liu X, Lin Y, Zhang H, Li M, Fang H, Liu J (2013) Profiling of StvacINV1 expression in regulation to acid invertase activity and sugar accumulation in potato cold-stored tubers. Potato Res 56:157–165
Pavek MJ, Knowles NR (2016) WSU potato cultivar yield and postharvest quality evaluations for 2015. Washington State University Special Report. (http://potatoes.wsu.edu/wp-content/uploads/2016/01/Potato-Cultivar-Yield-and-Postharvest-Quality-Evaluations-Research-Edition-2015.pdf)
Pavek JJ, Corsini DL, Love SL, Hane DC, Holm DG, Iritani WM, James SR, Martin MW, Mosley AR, Ojala JC, Shager CE, Thronton RE (1992) Ranger Russet: a long russet potato variety for processing and fresh market with improved quality, disease resistance, and yield. Am J Potato Res 69:483–488
Pressey R, Shaw R (1966) Effect of temperature on invertase, invertase inhibitor, and sugars in potato tubers. Plant Physiol 41:1657–1661
Reimholz R, Geiger M, Haake V, Deiting U, Kruase KP, Sonnewald U, Stitt M (1997) Potato plants contain multiple forms of sucrose phosphate synthase, which differ in their tissue distributions, their levels during development, and their responses to low temperature. Plant Cell Environ 20:291–305
Richardson DL, Davies HV, Ross HA, Mackay GR (1990) Invertase activity and its relation to hexose accumulation in potato tubers. J Exp Bot 41:95–99
Schippers PA (1977) The rate of respiration of potato tubers during storage 1. Review of literature. Potato Res 20:173–188
Smirnoff N (1993) The role of active oxygen in the response of plants to water-deficit and desiccation. New Phytol 125:27–58
Smith AM, Zeeman SC, Smith SM (2005) Starch degradation. Annu Rev Plant Biol 56:73–98
Sowokinos JR (2001a) Biochemical and molecular control of cold-induced sweetening in potatoes. Am J Potato Res 78:221–236
Sowokinos JR (2001b) Allele and isozyme patterns of UDP-glucose pyrophosphorylase as a marker for cold-sweetening resistance in potatoes. Am J Potato Res 78:57–64
Sowokinos JR (2007) Internal physiological disorders and nutritional and compositional factors that affect market quality. In: Vreugdenhil D (ed) Potato biology and biotechnology advances and perspectives. Elsevier, Amsterdam, pp 501–523
Stadler RH, Scholz G (2004) Acrylamide: an update on current knowledge in analysis, levels in food, mechanisms of formation, and potential strategies of control. Nutr Rev 62:449–466
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert MC, Riediker S (2002) Acrylamide from Maillard reaction products. Nature 419:449–450
Stark JC, Novy RG, Whitworth JL et al (2016) Mountain Gem Russet: a potato variety with high early and full season yield potential and excellent fresh market and early processing characteristics. Am J Potato Res 93:158–171
Steup M (1990) Starch degrading enzymes. In: Lea PJ (ed) Methods in plant biochemistry, vol 3. Elsevier, Amsterdam, pp 103–128
Stevenson WR, Loria R, Franc GD, Weingartner DP (2001) Physiological disorders of tubers: internal symptoms. In: Stevenson WR, Loria R, Franc GD, Weingartner DP (eds) Compendium of potato diseases, 2nd edn. APS Press, St. Paul
Struik PC, Ewing EE (1995) Crop Physiology of potato (Solanum tuberosum): responses to photoperiod and temperature relevant to crop modeling. In: Haverkort AJ, MacKerron DKL (eds) Potato ecology and modelling of crop under conditions of limiting growth, current issues in production ecology. Springer, Netherlands, pp 19–40
Tareke E, Rydberg P, Karlsson P, Eriksson P, Törnquist M (2000) Acrylamide: a cooking carcinogen? Chem Res Toxicol 13:517–522
Tareke E, Rydberg P, Karlsson P, Eriksson P, Törnquist M (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 50:4998–5006
Tauzin AS, Sulzenbacher G, Lafond M, Desseaux V, Reca IB, Perrier J, Bellincampi D, Fourquet P, Leveque C, Giardina T (2014) Functional characterization of a vacuolar invertase from Solanum lycopersicum: post-translational regulation by N-glycosylation and proteinaceous inhibitor. Biochimie 101:39–49
Thompson AL, Love SL, Sowokinos JR, Thornton MK, Shock CC (2008) Review of sugar end disorder (Solanum tuberosum L.). Am J Potato Res 85:375–386
Thornton ML, Buhrig W, Olson N (2010) The relationship between soil temperature and sugar ends in potato. Am J Potato Res 53:289–296
Timlin D, Lutfor RSM, Baker J, Reddy VR, Fleisher D, Quebedeaux B (2006) Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron J 98:1195–1203
Wiltshire JJJ, Cobb AH (1996) A review of the physiology of tuber dormancy. Ann Appl Biol 129:553–569
Wolf S, Marani A, Rudich J (1991) Effect of temperature on carbohydrate metabolism in potato plants. J Exp Bot 65:179–185
Yamaguchi M, Timm H, Spurr AR (1964) Effects of soil temperature on growth and nutrition of potato plants and tuberization, composition, and periderm structure of tubers. Proc Am Soc Hortic Sci 84:412–423
Zeeman SC, Thorneycroft D, Schupp N, Chapple A, Weck M, Dustan H, Haldimann P, Bechtold N, Smoth AM, Smith SM (2004) Plastidial α-glucan phosphorylase is not required for starch degradation in Arabidopsis leaves but has a role in the tolerance of abiotic stress. Plant Physiol 135:849–958
Zhu X, Richael C, Chamberlain P, Busse JS, Bussan AJ, Jiang J, Bethke PC (2014) Vacuolar invertase gene silencing in potato (Solanum tuberosum L.) improves process quality by decreasing the frequency of sugar-end defects. PLoS One 9:e93381. doi:10.1371/journal.pone.0093381
Zommick DH, Kumar GNM, Knowles LO, Knowles NR (2013) Translucent tissue defect in potato (Solanum tuberosum L.) tubers is associated with oxidative stress accompanying an accelerated aging phenotype. Planta 238:1125–1145
Zommick DH, Knowles LO, Pavek MJ, Knowles NR (2014) In-season heat stress compromises postharvest quality and low-temperature sweetening resistance in potato (Solanum tuberosum L.). Planta 239:1243–1263
Zrenner R, Schuler K, Sonnewald U (1996) Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198:246–252
Acknowledgements
We gratefully acknowledge financial support from the USDA-ARS, Northwest Potato Research Consortium and the Washington State Potato Commission. We thank the J.R. Simplot Company, Boise, ID for providing Innate® Russet Burbank (W8) seed potatoes and Dr. Richard G. Novy (USDA-ARS, Aberdeen, ID) for providing tubers of EGA09702-2.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herman, D.J., Knowles, L.O. & Knowles, N.R. Heat stress affects carbohydrate metabolism during cold-induced sweetening of potato (Solanum tuberosum L.). Planta 245, 563–582 (2017). https://doi.org/10.1007/s00425-016-2626-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-016-2626-z