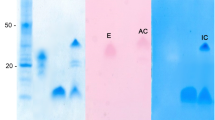
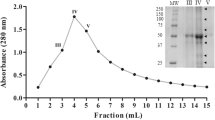
Abstract
A novel chymotrypsin inhibitor, detected in the endosperm of Triticum aestivum, was purified and characterized with respect to the main physical–chemical properties. On the basis of its specificity, this inhibitor was named WCI (wheat chymotrypsin inhibitor). WCI is a monomeric neutral protein made up of 119 residues and molecular mass value of 12,933.40 Da. Automated sequence and mass spectrometry analyses, carried out on several samples of purified inhibitor, evidenced an intrinsic molecular heterogeneity due to the presence of the isoform [des-(Thr)WCI], accounting for about 40% of the total sample. In vitro, WCI acted as a strong inhibitor of bovine pancreatic chymotrypsin as well as of chymotryptic-like activities isolated from the midgut of two phytophagous insects, Helicoverpa armigera (Hüb.) and Tenebrio molitor L., respectively. No inhibitory activities were detected against bacterial subtilisins, bovine pancreatic trypsin, porcine pancreatic elastase or human leukocyte elastase. The primary structure of WCI was significantly similar (45.7–89.1%) to those of several proteins belonging to the cereal trypsin/α-amylase inhibitor super-family and showed the typical sequence motif of this crowed protein group. The cDNA of the inhibitor (wci-cDNA) was isolated from wheat immature caryopses and employed to obtain a recombinant product in E. coli. Experimental evidences indicated that the recombinant inhibitor was localized in the inclusion bodies from which it was recovered as soluble and partially active protein by applying an appropriate refolding procedure. WCI reactive site localization, as well as its inhibitory specificity, was investigated by molecular modeling approach.






Similar content being viewed by others
Abbreviations
- BTEE:
-
N-Benzoyl-l-tyrosine ethyl ester
- CNBr:
-
Cyanogen bromide
- DTT:
-
Dithiothreitol
- IPTG:
-
Isopropyl-β-d-1-thiogalactopyranoside
- MALDI-TOF:
-
Matrix-assisted laser desorption/ionisation-time of flight
- PAGE:
-
Polyacrylamide gel electrophoresis
- PCR:
-
Polymerase chain reaction
- SDS:
-
Sodium dodecyl sulphate
- TAME:
-
N-α-p-Toluene-sulphonyl-l-arginine methyl ester
- TFA:
-
Trifluoroacetic acid
- TLCK:
-
N-p-tosyl-l-Lysine chloromethyl ketone
- TPCK:
-
N-p-tosyl-l-Phenylalanine chloromethyl ketone
References
Alfonso-Rubi J, Ortego F, Castanera P, Carbonero P, Diaz I (2003) Transgenic expression of trypsin inhibitor CMe from barley in indica and japonica rice, confers resistance to the rice weevil Sitophilus oryzae. Transgenic Res 12:23–31
Barber D, Sàncez-Monge R, Garcia-Olmedo F, Salcedo G, Mendez E (1986) Evolutionary implications of sequential homologies among members of the trypsin/α-amylase inhibitor family (CM proteins) in wheat and barley. Biochim Biophys Acta 873:147–151
Behnke CA, Yee VC, Trong IL, Pedersen LC, Stenkamp RE, Kim SS, Reeck GR, Teller DC (1998) Structural determinants of the bifunctional corn Hageman factor inhibitor: X-ray crystal structure at 1.95 Å resolution. Biochemistry 37:15277–15288
Birk Y (2003) Plant protease inhibitors: significance in nutrition, plant protection, cancer prevention and genetic engineering. Springer, Berlin
Bode W, Huber R (1992) Natural protein proteinase inhibitors and their interaction with proteinases. Eur J Biochem 204:433–451
Boisen S, Djurtoft R (1981) Trypsin inhibitor from wheat endosperm: purification and characterization. Cereal Chem 58:460–463
Bowman DE (1948) Further identification of bean trypsin inhibiting factors. Arch Biochem Biophys 16:109–113
Buonocore V, Gramenzi F, Pace W, Petrucci T, Poerio E, Silano V (1980) Interaction of wheat monomeric and dimeric protein inhibitors with alpha-amylase from yellow mealworm (Tenebrio molitor L. larva). Biochem J 187:637–645
Cha S (1975) Tight-binding inhibitors-I. Kinetic behavior. Biochem Pharmacol 24:2177–2185
Del Vecchio Blanco F, Cafaro V, Di Maro A, Scognamiglio R, Siniscalco G, Parente A, Di Donato A (1998) A recombinant ribosome-inactivating protein from the plant Phytolacca dioica L. produced from a synthetic gene. FEBS Lett 437:241–245
Di Gennaro S, Ficca AG, Panichi D, Poerio E (2005) cDNA cloning and heterologous expression of a wheat proteinase inhibitor of subtilisin and chymotrypsin (WSCI) that interferes with digestive enzymes of insect pests. Biol Chem 386:383–389
Di Maro A, Ferranti P, Mastronicola M, Polito L, Bolognesi A, Stirpe F, Malorni A, Parente A (2001) Reliable sequence determination of ribosome- inactivating proteins by combining electrospray mass spectrometry and Edman degradation. J Mass Spectrom 36:38–46
Garcia-Casado G, Sanchez-Monge R, Lopez-Otin C, Salcedo G (1994) Rye inhibitors of animal alpha-amylases show different specificities, aggregative properties and IgE-binding capacities than their homologues from wheat and barley. Eur J Biochem 224:525–531
Garcia-Olmedo F, Salcedo v, Sanchez-Monge R, Gomez L, Royo J, Carbonero P (1987) Plant proteinaceous inhibitors of proteinases and alpha-amylases. Oxford Surv Plant Mol Cell Biol 4:275–334
Gautier MF, Alary R, Joudrier P (1990) Cloning and characterization of a cDNA encoding the wheat (Triticum durum Desf.) CM16 protein. Plant Mol Biol 14:313–322
Gourinath S, Alam N, Srinivasan A, Betzel C, Singh TP (2000) Structure of the bifunctional inhibitor of trypsin and α-amylase from ragi seeds at 2.2 Å resolution. Acta Crystallogr D Biol Crystallogr 56:287–293
Janoff A (1969) Alanine p-nitrophenyl esterase activity of human leucocyte granules. Biochem J 114:157–159
Oda Y, Matsunaga T, Fukuyama K, Miyazaki T, Morimoto T (1997) Tertiary and quaternary structures of 0.19 α-amylase inhibitor from wheat kernel determined by X-ray analysis at 2.06 Å resolution. Biochemistry 36:13503–13511
Odani S, Koide T, Ono T (1983) A possible evolutionary relationship between plant trypsin inhibitor, alpha-amylase inhibitor, and mammalian pancreatic secretory trypsin inhibitor (Kazal). J Biochem 1993:1701–1704
Parente A, Merrifield B, Geraci G, D’Alessio G (1985) Molecular basis of superreactivity of cysteine residues 31 and 32 of seminal ribonuclease. Biochemistry 24:1098–1104
Poerio E, Carrano L, Carzillo AM, Buonocore V (1989) A trypsin inhibitor from the water-soluble protein fraction of wheat kernel. Phytochemistry 28:1307–1311
Poerio E, Di Gennaro S, Di Maro A, Farisei F, Ferranti P, Parente A (2003) Primary structure and reactive site of a novel wheat proteinase inhibitor of subtilisin and chymotrypsin. Biol Chem 384:295–304
Prescott A, Martin C (1987) A rapid method for the quantitative assessment of levels of specific mRNAs in plants. Plant Mol Biol Rep 4:219–224
Rawlings ND, Tolle DP, Barrett AJ (2004a) Evolutionary families of peptidase inhibitors. Biochem J 378:705–716
Rawlings ND, Barrett AJ, Bateman A (2004b) MEROPS: the peptidase database. Nucleic Acids Res 38:D227–D233
Richardson M (1991) Seed storage proteins: the enzyme inhibitors. In: Rogers LJ (ed) Methods in plant biochemistry, vol 5. Academic Press, New York, pp 259–305
Robinson LH, Juttner J, Milligan A, Lahnstein J, Eglinton JK, Evans DE (2007) The identification of a barley haze active protein that influences beer haze stability: cloning and characterisation of the barley SE protein as a barley trypsin inhibitor of the chloroform/methanol type. J Cereal Sci 45:343–352
Rose TM, Schultz ER, Henikoff JG, Pietrokovski S, McCallum CM, Henikoff S (1998) Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Res 26:1628–1635
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5:725–738
Ryan CA (1990) Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28:425–449
Schagger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Scudiero R, Capasso C, Del Vecchio-Blanco F, Savino G, Capasso A, Parente A, Parisi E (1995) Isolation and primary structure determination of a metallothionein from Paracentrotus lividus (Echinodermata, Echinoidea). Comp Biochem Phys B Biochem Mol Biol 111:329–336
Severino V, Chambery A, Vitiello M, Cantisani M, Galdiero S, Galdiero M, Malorni L, Di Maro A, Parente A (2010) Proteomic analysis of human U937 cell line activation mediated by Haemophilus influenzae type b P2 porin and its surface-exposed loop 7. J Proteome Res 9:1050–1062
Shewry PR (1995) Plant storage proteins. Biol Rev 70:375–426
Strobl S, Muhlhahn P, Bernstein R, Wiltscheck R, Maskos K, Wunderlich M, Huber R, Glockshuber R, Holak TA (1995) Determination of the three-dimensional structure of the bifunctional alpha-amylase/trypsin inhibitor from ragi seeds by NMR spectroscopy. Biochemistry 34:8281–8293
Strobl S, Maskos K, Betz M, Wiegand G, Huber R, Gomis-Ruth FX, Glockshuber R (1998) Crystal structure of yellow meal worm α-amylase at 1.64 Å resolution. J Mol Biol 278:617–628
Ussuf KK, Laxmi NH, Mitra R (2001) Proteinase inhibitors: plant-derived genes of insecticidal protein for developing insect-resistant transgenic plants. Curr Sci 80:847–853
Acknowledgments
This research was supported by the Grant COFIN 2006, from the Italian Ministero Istruzione, Università e Ricerca (MIUR) and funds from Università degli Studi della Tuscia and the Seconda Università degli Studi di Napoli. The post-doc fellowships to Dr. Natalia Bruni and Valeria Capuzzi were funded by the Università degli Studi della Tuscia. The post-doc fellowship to Dr. Francesca Tedeschi was funded by the Italian Ministero Istruzione, Università e Ricerca (MIUR) and Seconda Università degli Studi di Napoli, with the grant Fondo Sostegno Giovani, E.F. 2008.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was dedicated by Elia Poerio to the memory of Dr. Hiroshi Taniuchi, distinguished scientist of remarkable intellectual integrity.
The protein and nucleotide sequence data reported in this paper were deposited in the UniProt Knowledgebase and NBCI data bank under the following accession numbers: P83207 and AJ422078, respectively.
Rights and permissions
About this article
Cite this article
Di Maro, A., Farisei, F., Panichi, D. et al. WCI, a novel wheat chymotrypsin inhibitor: purification, primary structure, inhibitory properties and heterologous expression. Planta 234, 723–735 (2011). https://doi.org/10.1007/s00425-011-1437-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-011-1437-5