Abstract
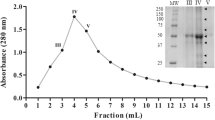
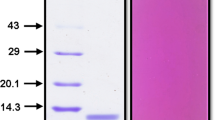
A Kunitz-type protease inhibitor (OPI, okra protease inhibitor) has been purified from okra (Abelmoschus esculentus) seeds by a combination of ammonium sulfate precipitation, anion-exchange chromatography and reverse-phase high-performance liquid chromatography. The protein shows an apparent mass of 21 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing condition. OPI exhibits inhibitory activity against trypsin. Analysis of the far-UV circular dichroism spectrum showed that the protein contains ~39% β-sheets but only ~5% α-helices. The protein is thermally quite stable, and exhibits a cooperative thermal unfolding transition at ~70°C, as determined by circular dichroism spectroscopy and differential scanning fluorimetry. De novo sequencing of OPI by nanoESI-Q-ToF mass spectrometry (MS) allowed the assignment of about 83% of its primary structure, which indicated that the protein shares 43% sequence identity with a putative 21 kDa trypsin inhibitor from Theobroma bicolor. An intramolecular disulfide linkage between Cys149 and Cys156 was also detected. The protein showed ~24 and ~25% sequence identity with α-amylase/subtilisin inhibitor from barley and soybean (Kunitz) trypsin inhibitor, respectively. Comparative structure modeling of OPI revealed a structural fold similar to other Kunitz-type TIs. The presence of Cys149–Cys156 disulfide bond as detected by MS and a second disulfide bond connecting Cys44–Cys91, conserved in all Kunitz-type TIs, is also identified in the model.









Similar content being viewed by others
Abbreviations
- ACN:
-
acetonitrile
- BAEE:
-
N-α-benzoyl-l-arginine ethyl ester
- CID:
-
collision induced dissociation
- CD:
-
circular dichroism
- DSF:
-
differential scanning fluorimetry
- DTT:
-
dithiothreitol
- OPI:
-
okra protease inhibitor
- PI:
-
protease inhibitor
- RP-HPLC:
-
reverse-phase high-performance liquid chromatography
- RT:
-
retention time
- SDS-PAGE:
-
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SKTI:
-
soybean Kunitz-trypsin inhibitor
- TFA:
-
trifluoroacetic acid
- TI:
-
trypsin inhibitor
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W and Lipman DJ 1997 Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402
Azarkan M, Dibiani R, Goormaghtigh E, Raussens V and Baeyens-Volant D 2006 The papaya Kunitz-type trypsin inhibitor is a highly stable β-sheet glycoprotein. Biochim. Biophys. Acta 1764 1063–1072
Bhattacharyya A and Babu CR 2009 Purification and biochemical characterization of a serine proteinase inhibitor from Derris trifoliata Lour. seeds: Insight into structural and antimalarial features. Phytochemistry 70 703–712
Bobbili KB, Pohlentz G, Narahari A, Sharma K, Surolia A, Mormann M and Swamy MJ 2018 Coccinia indica agglutinin, a 17 kDa PP2 like phloem lectin: Affinity purification, primary structure and formation of self-assembled filaments. Int. J. Biol. Macromol. 108 1227–1236
Datta D and Swamy MJ 2017 Fluorescence and circular dichroism studies on the accessibility of tryptophan residues and unfolding of a jacalin-related α-d-galactose-specific lectin from mulberry (Morus indica). J. Photochem. Photobiol. B 170 108–117
Datta D, Pohlentz G, Schulte M, Kaiser M, Goycoolea FM, Müthing J, Mormann M and Swamy MJ 2016 Physico-chemical characteristics and primary structure of an affinity-purified α-D-galactose-specific, jacalin-related lectin from the latex of mulberry (Morus indica). Arch. Biochem. Biophys. 609 59–68
De Leo F, Volpicella M, Licciulli F, Liuni S, Gallerani R and Ceci LR 2002 PLANT-PIs: A database for plant protease inhibitors and their genes. Nucleic Acids Res. 30 347–348
García-Olmedo F, Salcedo G, Sánchez-Monge R, Gómez L, Royo J and Carbonero P 1987 Plant proteinaceous inhibitors of proteinases and alpha-amylases. Oxford Surv. Plant Mol. Cell Biol. 4 275–334
Habib H and Fazili KM 2007 Plant protease inhibitors: A defense strategy in plants. Biotechnol. Mol. Biol. Rev. 2 68–85
Kessler A and Baldwin IT 2002 Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol. 53 299–328
Kochhar S, Gartenmann K and Juillerat MA 2000 Primary structure of the abundant seed albumin of Theobroma cacao by mass spectrometry. J. Agric. Food Chem. 48 5593–5599
Kumar G, Pohlentz G, Schulte M, Mormann M and Kumar NS 2014 Jack bean α-mannosidase: amino acid sequencing and N-glycosylation analysis of a valuable glycomics tool. Glycobiology 24 252–261
Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685
Laskowski M Jr and Kato I 1980 Protein inhibitors of proteinases. Annu. Rev. Biochem. 49 593–626
Laskowski M and Qasim MA 2000 What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim. Biophys. Acta. 1477 324–337
Lawrence PK and Koundal KR 2002 Plant protease inhibitors in control of phytophagous insects. Electron. J. Biotechnol. 5 93–109
Lopez-Otin C and Bond JS 2008 Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 283 30433–30437
Mormann M, Eble J, Schwöppe C, Mesters RM, Berdel WE, Peter-Katalinić J and Pohlentz G 2008 Fragmentation of intra-peptide and inter-peptide disulfide bonds of proteolytic peptides by nanoESI collision-induced dissociation. Anal. Bioanal. Chem. 392 831–838
Mosolov VV and Valueva TA 2005 Proteinase inhibitors and their function in plants: A review. Appl. Biochem. Microbiol. 41 227–246
Ogata F, Imamura H, Hirayama K and Makisumi S 1986 Purification and characterization of four trypsin inhibitors from seeds of okra, Abelmoschus esculentus L. Agric. Biol. Chem. 50 2325–2333
Oliva MLV, Silva MC, Sallai RC, Brito MV and Sampaio MU 2010 A novel subclassification for Kunitz proteinase inhibitors from leguminous seeds. Biochimie. 92 1667–1673
Pohlentz G, Marx K and Mormann M 2016 Characterization of protein N-glycosylation by analysis of ZIC-HILIC-enriched intact proteolytic glycopeptides; In: Methods in molecular biology, proteomics in systems biology: Methods and protocols (ed) Reinders J Vol 1394, (Heidelberg: Springer) Chapter 12, pp 163–179
Ramasarma PR, Appu Rao AG and Rao DR 1995 Role of disulfide linkages in structure and activity of proteinase inhibitor from horsegram (Dolichos biflorus). Biochim. Biophys. Acta 1248 35–42
Read SM and Northcote DH 1983 Subunit structure and interactions of the phloem proteins of Cucurbita maxima (pumpkin). Eur. J. Biochem. 134 561–569
Roy A, Shrivastava SL and Mandal SM 2014 Functional properties of Okra Abelmoschus esculentus L.(Moench): Traditional claims and scientific evidences. Plant Sci. Today 1 121–130
Roychaudhuri R, Sarath G, Zeece M and Markwell J 2003 Reversible denaturation of the soybean Kunitz trypsin inhibitor. Arch. Biochem. Biophys. 412 20–26
Ryan CA 1990 Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu. Rev. Phytopathol. 28 425–449
Šali A and Blundell TL 1993 Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234 779–815
Shevchenko A, Tomas H, Havli J, Olsen JV and Mann M 2006 In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1 2856–2860
Shukla E, Agrawal SB and Gaikwad SM 2017 Conformational and functional transitions and in silico analysis of a serine protease from Conidiobolus brefeldianus (MTCC 5185). Int. J. Biol. Macromol. 98 387–397
Sievers F and Higgins DG 2014 Clustal omega. Curr. Protoc. Bioinf. 48 3–13
Song HK and Suh SW 1998 Kunitz-type soybean trypsin inhibitor revisited: Refined structure of its complex procine trypsin reveals an insight into the interaction between a homologous inhibitor from Erythrina caffra and tissue-type plasminogen activator. J. Mol. Biol. 275 347–363
Spencer ME and Hodge R 1991 Cloning and sequencing of the cDNA encoding the major albumin of Theobroma cacao. Planta 183 528–535
Sreerama N and Woody RW 2000 Estimation of protein secondary structure from CD spectra: comparison of CONTIN, SELCON and CDSSTR methods with an expanded reference set. Anal. Biochem. 287 252–260
Sultan NAM, Kenoth R and Swamy MJ 2004 Purification, physicochemical characterization, saccharide specificity, and chemical modification of a Gal/GalNAc specific lectin from the seeds of Trichosanthes dioica. Arch. Biochem. Biophys. 432 212–221
Tai H, McHenry L, Fritz PJ and Furtek DB 1991 Nucleic acid sequence of a 21 kDa cocoa seed protein with homology to the soybean trypsin inhibitor (Kunitz) family of protease inhibitors. Plant Mol. Biol. 16 913–915
Thyrock A, Ossendorf E, Stehling M, Kail M, Kurtz T, Pohlentz G, Waschbüsch D, Eggert S, Formstecher E, Müthing J and Dreisewerd K 2013 A new Mint1 isoform, but not the conventional Mint1, interacts with the small GTPase Rab6. PLOS One 8 e64149
Yanes O, Villanueva J, Querol E and Aviles FX 2007 Detection of non-covalent protein interactions by ‘intensity fading’ MALDI-TOF mass spectrometry: Applications to proteases and protease inhibitors. Nat. Protoc. 2 119–130
Zhou D, Lobo YA, Batista IF, Marques-Porto R, Gustchina A, Oliva ML and Wlodawer A 2013 Crystal structures of a plant trypsin inhibitor from Enterolobium contortisiliquum (EcTI) and of its complex with bovine trypsin. PLOS One 8 e62252
Zhu-Salzman K and Zeng R. 2015 Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 60 233–252
Acknowledgements
This work was supported by a research grant from the Department of Biotechnology (India) to MJS. We thank the University Grants Commission (India) for its support through the UPE-II grant to the University of Hyderabad and the CAS program to the School of Chemistry. Support from the Department of Science and Technology under the FIST and PURSE programs is gratefully acknowledged. DD was supported by Senior Research Fellowship from the Council of Scientific and Industrial Research (CSIR), India. The authors are thankful to Nano Temper technologies, Bangalore, India for the use of Prometheus NT.48 instrument and their application specialist, Ms. Saji Menon for help with the DSF experiments. This project was carried out under IRTG-MCGS (GRK 1549) financed by DFG in Germany and UGC in India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by BJ Rao.
Corresponding editor: BJ Rao
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Datta, D., Pohlentz, G., Mondal, S. et al. Macromolecular properties and partial amino acid sequence of a Kunitz-type protease inhibitor from okra (Abelmoschus esculentus) seeds. J Biosci 44, 35 (2019). https://doi.org/10.1007/s12038-019-9859-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-019-9859-5