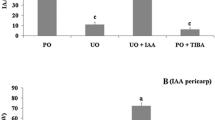
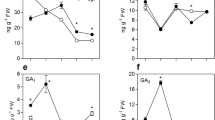
Abstract
We investigated the role of gibberellins (GAs) in the phenotype of parthenocarpic fruit (pat), a recessive mutation conferring parthenocarpy in tomato (Solanum lycopersicum L.). Novel phenotypes that parallel those reported in plants repeatedly treated with gibberellic acid or having a GA-constitutive response indicate that the pat mutant probably expresses high levels of GA. The retained sensitivity to the GA-biosynthesis inhibitor paclobutrazol reveals that this condition is dependent on GA biosynthesis. Expression analysis of genes encoding key enzymes involved in GA biosynthesis shows that in normal tomato ovaries, the GA20ox1 transcript is in low copy number before anthesis and only pollination and fertilization increase its transcription levels and, thus, GA biosynthesis. In the unpollinated ovaries of the pat mutant, this mechanism is de-regulated and GA20ox1 is constitutively expressed, indicating that a high GA concentration could play a part in the parthenocarpic phenotype. The levels of endogenous GAs measured in the floral organs of the pat mutant support such a hypothesis. Collectively, the data indicate that transcriptional regulation of GA20ox1 mediates pollination-induced fruit set in tomato and that parthenocarpy in pat results from the mis-regulation of this mechanism. As genes involved in the control of GA synthesis (LeT6, LeT12 and LeCUC2) and response (SPY) are also altered in the pat ovary, it is suggested that the pat mutation affects a regulatory gene located upstream of the control of fruit set exerted by GAs.






Similar content being viewed by others
Abbreviations
- CUC2:
-
Cup-shaped cotyledon2
- DAP:
-
Days after pollination
- GA:
-
Gibberellin
- GA3 :
-
Gibberellic acid
- GA20ox:
-
GA 20-oxidase
- GA3ox:
-
GA 3-oxidase
- GC-MS/MS:
-
Chromatography–tandem mass spectrometry
- HAE:
-
Hours after emasculation
- HAP:
-
Hours after pollination
- HBP:
-
Hours before pollination
- HP:
-
Hand-pollinated
- IAA:
-
Indole-3-acetic acid
- KNOX :
-
Knotted-like homeobox genes
- OP:
-
Open-pollinated
- PAC:
-
Paclobutrazol
- Pat:
-
Parthenocarpic fruit
- RT:
-
Reverse transcription
- SPY :
-
Spindly
- WT:
-
Wild type
References
Abdul KS, Harris GP (1978) Control of flower number in the first inflorescenze of tomato (Lycopersicon esculentum Mill.): the role of gibberellins. Ann Bot 42:1361–1367
Aida M, Ishida T, Tasaka M (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570
Angenent GC, Stuurman J, Snowden KC, Koes R (2005) Use of Petunia to unravel plant meristem functioning. Trends Plant Sci 10:243–250
Ben-Cheikh W, Perez-Botella J, Tadeo FR, Talon M, Primo-Millo E (1997) Pollination increases gibberellin levels in developing ovaries of seeded varieties of Citrus. Plant Physiol 114:557–564
Beraldi D, Picarella ME, Soressi GP, Mazzucato A (2004) Fine mapping of the parthenocarpic fruit (pat) mutation in tomato. Theor Appl Genet 108:209–216
Fos M, Nuez F, García-Martínez J (2000) The gene pat-2, which induces natural parhenocarpy, alters the gibberellin content in unpollinated tomato ovaries. Plant Physiol 122:471–479
Fos M, Proaño K, Nuez F, García-Martínez JL (2001) Role of gibberellins in parthenocarpic fruit development induced by the genetic system pat-3/pat-4 in tomato. Physiol Plant 111:545–550
García-Martínez JL, López-Diaz I, Sánchez-Beltrán MJ, Phillips AL, Ward DA, Gaskin P, Hedden P (1997) Isolation and transcript analysis of gibberellin 20-oxidase genes in pea and bean in relation to fruit development. Plant Mol Biol 33:1073–1084
Gillaspy G, Ben-David H, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5:1439–1451
Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM (2006) AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 18:1873–1886
Gorguet B, van Heusden AW, Lindhout P (2005) Parthenocarpic fruit development in tomato. Plant Biol 7:131–139
Greb T, Schmitz G, Theres K (2002) Isolation and characterization of the Spindly homologue from tomato. J Exp Bot 53:1829–1830
Harrison AL (1955) New “mouse-eared” mutant from var Rutgers. Rep Tomato Genet Coop 5:18
Hay A, Kaur H, Phillips A, Hedden P, Hake S, Tsiantis M (2002) The gibberellin pathway mediates KNOTTED1-type homeobox function in plants with different body plans. Curr Biol 12:1557–1565
Hisamatsu T, King RW, Helliwell CA, Koshioka M (2005) The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol 138:1106–1116
Ishida T, Aida M, Takada S, Tasaka M (2000) Involvement of CUP-SHAPED COTYLEDON genes in gynoecium and ovule development in Arabidopsis thaliana. Plant Cell Physiol 41:60–67
Jacobsen SE, Olszewski NE (1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5:887–896
Jacobsen SE, Binkowski KA, Olszewski NE (1996) SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA 93:9292–9296
Janssen B-J, Williams A, Chen J-J, Mathern J, Hake S, Sinha N (1998a) Isolation and characterization of two knotted-like homeobox genes from tomato. Plant Mol Biol 36:417–425
Janssen B-J, Lund L, Sinha N (1998b) Overexpression of a homeobox gene, LeT6, reveals indeterminate features in the tomato compound leaf. Plant Physiol 117:771–786
Jasinski S, Piazza P, Craft J, Hay A, Wooley L, Rieu I, Phillips A, Hedden P, Tsiantis M (2005) KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15:1560–1565
Kamiya Y, García-Martínez JL (1999) Regulation of gibberellin biosynthesis by light. Curr Opin Plant Biol 2:398–403
Kang H-G, Jun S-H, Kim J, Kawaide H, Kamiya Y, An G (1999) Cloning and molecular analyses of a gibberellin 20-oxidase gene expressed specifically in developing seeds of watermelon. Plant Physiol 121:373–382
Kataoka K, Uemachi A, Yazawa S (2003) Fruit growth and pseudoembryo development affected by uniconazole, an inhibitor of gibberellin biosynthesis, in pat-2 and auxin-induced parthenocarpic tomato fruits. Sci Hortic 98:9–16
Kataoka K, Okita H, Uemachi A, Yazawa S (2004) A pseudoembryo highly stainable with toluidine blue may induce fruit growth of parthenocarpic tomato. Acta Hortic 637:213–221
Kerstetter RA, Laudencia-Chingcuanco D, Smith LG, Hake S (1997) Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124:3045–3054
King PJ (1988) Plant hormone mutants. Trends Genet 4:157–162
Koltunow AM, Vivian-Smith A, Tucker MR, Paech N (2002) The central role of the ovule in apomixis and parthenocarpy. In: O’Neill SD, Roberts JA (eds) Plant reproduction. Academic, Sheffield, pp 221–256
Koshioka M, Nishijima T, Yamazaki H, Nonaka M, Mander LN (1994) Analysis of gibberellins in growing fruits of Lycopersicon esculentum after pollination or treatment with 4-chlorophenoxyacetic acid. J Hortic Sci 69:171–179
Mapelli S, Frova C, Torti G, Soressi GP (1978) Relationship between set, development and activities of growt regulators in tomato fruits. Plant Cell Physiol 19:1281–1288
Mazzucato A, Taddei AR, Soressi GP (1998) The parthenocarpic fruit (pat) mutant of tomato (Lycopersicon esculentum Mill.) sets seedless fruits and has aberrant anther and ovule development. Development 125:107–114
Mazzucato A, Testa G, Biancari T, Soressi GP (1999) Effect of gibberellic acid treatments, environmental conditions, and genetic background on the expression of the parthenocarpic fruit mutation in tomato. Protoplasma 208:18–25
Nitsch J (1970) Hormonal factors in growth and development. In: AC Hulme (ed) The biochemistry of fruits and their products, vol II. Academic, London, pp 427–472
O’Neill SD (1997) Pollination regulation of flower development. Annu Rev Plant Physiol Plant Mol Biol 48:547–574
Oikawa T, Koshioka M, Kojima K, Yoshida H, Kawata M (2004) A role of OsGA20ox1, encoding an isoform of gibberellin 20-oxidase, for regulation of plant stature in rice. Plant Mol Biol 55:687–700
Olszewski N, Sun TP, Gubler F (2002) Gibberellin signaling: biosynthesis, catabolism, and response pathways. Plant Cell 14(Suppl):S61–S80
Ozga JA, Reinecke DM (2003) Hormonal interactions in fruit development. J Plant Growth Regul 22:73–81
Ozga JA, Yu J, Reinecke DM (2003) Pollination-, development-, and auxin-specific regulation of gibberellin 3β-hydroxilase gene expression in pea fruit and seeds. Plant Physiol 131:1137–1146
Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir D, Lifschitz E (1997) The dominant developmental mutants of tomato, Mouse-ear and Curl, are associated with distinct modes of abnormal transcriptional regulation of a Knotted gene. Plant Cell 9:2143–2158
Picciarelli P, Piaggesi L, Ceccarelli N, Guglielminetti L, Alpi A (1994) Gibberellins in suspensor, embryo and integuments from very young seeds of Phaseolus coccineus L. Plant Growth Regul 14:183–185
Ray A, Robinson-Beers K, Ray S, Baker SC, Lang JD, Preuss D, Milligan SB, Gasser CS (1994) Arabidopsis floral homeotic gene BELL (BEL1) controls ovule development through negative regulation of AGAMOUS gene (AG). Proc Natl Acad Sci USA 91:5761–5765
Rebers M, Kaneta T, Kawaide H, Yamaguchi S, Yang YY, Imai R, Sekimoto H, Kamiya Y (1999) Regulation of gibberellin biosynthesis genes during flower and early fruit development of tomato. Plant J 17:241–250
Ross JJ, O’Neill DP, Smith JJ, Kerckhoffs LHJ, Elliot RC (2000) Evidence that auxin promotes gibberellin A1 biosynthesis in pea. Plant J 21:547–552
Sawhney VK, Greyson RI (1971) Induction of multilocular ovary in tomato by gibberellic acid. J Am Soc Hortic Sci 96:196–198
Schwabe WW, Mills JJ (1981) Hormones and parthenocarpic fruit set. Hort Abstr 51:661–698
Schumacher K, Schmitt T, Rossberg M, Schmitz G, Theres K (1999) The lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc Natl Acad Sci USA 96:290–295
Solfanelli C, Ceron F, Paolicchi F, Giorgetti L, Geri C, Ceccarelli N, Kamiya Y, Picciarelli P (2005) Expression of two genes encoding gibberellin 2- and 3-oxidases in developing seeds of Phaseolus coccineus. Plant Cell Physiol 46:1116–1124
Sun TP, Gubler F (2004) Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol 55:197–223
Talon M, Koornneef M, Zeevaart JAD (1990) Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA 87:7983–7987
Talon M, Zacarias L, Primo-Millo E (1992) Gibberellins and parthenocarpic ability in developing ovaries of seedless mandarins. Plant Physiol 99:1575–1581
Tanaka-Ueguchi M, Itoh H, Oyama N, Koshioka M, Matsuoka M (1998) Over-expression of a tobacco homeobox gene, NTH15, decreases the expression of a gibberellin biosynthetic gene encoding GA 20-oxidase. Plant J 15:391–400
Testa G, Caccia R, Tilesi F, Soressi GP, Mazzucato A (2002) Sequencing and characterization of tomato genes putatively involved in fruit set and early development. Sex Plant Reprod 14:269–277
Varoquaux F, Blanvillain R, Delseny M, Gallois P (2000) Less is better: new approaches for seedless fruit production. Trends Biotechnol 18:233–242
Vivian-Smith A, Luo M, Chaudhury A, Koltunow A (2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128:2321–2331
Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech J-C, Bouzayen M (2005) The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 17:2676–2692
Acknowledgments
We thank Dr. Francesca Tilesi (Dipartimento di Biologia Cellulare e dello Sviluppo, Università La Sapienza, Roma, Italy) and Dr. Dario Beraldi (Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, UK) for their contribution to the early stages in this work; Pietro Mosconi (Dipartimento di Agrobiologia e Agrochimica, Università della Tuscia, Viterbo, Italy) for expert technical assistance and Dr. Giovanna Frugis (Istituto di Biologia e Biotecnologia Agraria, Roma, Italy), Dr. Theo Lange (Institut für Pflanzenbiologie, Technischen Universität Braunschweig, Braunschweig, Germany) and two anonymous reviewers for their valuable comments on the manuscript and Jacqueline Scarpa for help with the English form of the manuscript. This work was supported by the funds from the University of Tuscia for Scientific Research, project “Ruolo delle gibberelline nell’allegagione e nello sviluppo del frutto in pomodoro”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2007_533_MOESM1_ESM.ppt
Fig. S1 The principal pathways of gibberellin (GA) metabolism in higher plants, including the non-early-13-hydroxylated pathway (left branch) and the early-13-hydroxylated pathway (right branch). Arrows connect the precursor with the product of each step and circled numbers refers to the enzymes catalysing the reactions as follows: (1) ent-copalyl diphosphate synthase; (2) ent-kaurene synthase; (3) ent-kaurene 19-oxidase; (4) kaurenoic acid oxidase; (5) GA 13-hydroxylase; (6) GA 20-oxidase (GA20ox); (7) GA 3β-hydroxylase (GA3ox); (8) GA 2-oxidase (GA2ox). Those components of the early-13-hydroxylated pathway that have been monitored in this study are highlighted. (Modified from Hedden and Phillips, 2000). GGPP, geranylgeranyl pyrophosphate (DOC 39 kb)
425_2007_533_MOESM2_ESM.ppt
Fig. S2 Expression analysis of genes involved in GA synthesis and response in WT (white bars) and pat mutant (black bars) tomato ovaries at the time of flower opening (stage 3). RNA was isolated and reverse transcribed using oligo(dT) and Moloney murine leukemia virus-reverse transcriptase. The cDNAs generated were subsequently used in a 25 ml PCR reaction in the presence of primers specific for the studied genes or the ACTIN control. The RT-PCR products were separated on 1.5% (w/v) agarose gels stained with ethidium bromide. Expression data are reported as estimates of relative mRNA amount derived from the ratio between the quantitative values of PCR band intensity for the target gene and the actin control measured by the Gel Analyzer procedure of the ImageJ software (http://rsb.info.nih.gov/ij/). Data points are means of three biological replicates ±SE and * indicates significant differences for P£0.05 between the WT and pat mutant ovaries after one-way analysis of variance (DOC 37 kb)
425_2007_533_MOESM3_ESM.doc
Table S1. Sets of RT-PCR primers and PCR conditions used to amplify tomato gene-specific regions of coding sequences involved in GA biosynthesis and response (DOC 33 kb)
Rights and permissions
About this article
Cite this article
Olimpieri, I., Siligato, F., Caccia, R. et al. Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis . Planta 226, 877–888 (2007). https://doi.org/10.1007/s00425-007-0533-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-007-0533-z