Abstract
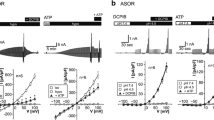
Recently, a novel type of anion channel activated by extracellular acidification has been found in a variety of mammalian cell types. However, the role of this acid-sensitive outwardly rectifying (ASOR) anion channel is not known. In human epithelial HeLa cells, reduction in extracellular pH below 5 rapidly activated anion-selective whole-cell currents. The currents exhibited strong outward rectification, activation kinetics at positive potentials, low-field anion selectivity, and sensitivity to 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and phloretin. When outside-out patches were exposed to acidic bathing solution, single-channel events of the anion channel could be observed. The unitary conductance was 4.8 pS in the voltage range between −80 and +80 mV. The single-channel activity prominently increased with depolarization and was suppressed by DIDS or phloretin. After 1-h incubation in acidic solution (pH 4.5), a significant population of HeLa cells suffered from necrotic cell injury characterized by stainability with propidium iodide and lack of caspase-3 activation. Upon exposure to acidic solution, HeLa cells exhibited immediate, persistent swelling. Both the necrotic volume increase and cell injury induced by extracellular acidification were inhibited by DIDS or phloretin. Therefore, it is concluded that the ASOR anion channel is involved in the genesis of necrotic cell injury induced by acidosis in human epithelial cells.






Similar content being viewed by others
References
Argenzio RA, Eisemann J (1996) Mechanisms of acid injury in porcine gastroesophageal mucosa. Am J Vet Res 57:564–573
Auzanneau C, Thoreau V, Kitzis A, Becq F (2003) A novel voltage dependent chloride current activated by extracellular acidic pH in cultured rat Sertoli cells. J Biol Chem 278:19230–19236
Baron A, Pacaud P, Loirand G, Mironneau C, Mironneau J (1991) Pharmacological block of Ca2+-activated Cl− current in rat vascular smooth muscle cells in short-term primary culture. Pflügers Arch 419:553–558
Barros LF, Hermosilla T, Castro J (2001) Necrotic volume increase and the early physiology of necrosis. Comp Biochem Physiol 130:401–409
Chen MF, Chen TY (2001) Different fast-gate regulation by external Cl− and H+ of the muscle-type ClC chloride channels. J Gen Physiol 118:23–32
Chesler M, Kaila K (1992) Modulation of pH by neuronal activity. Trends Neurosci 15:396–402
Davis JN, Antonawich FJ (1997) Role of apoptotic proteins in ischemic hippocampal damage. Ann N Y Acad Sci 835:309–320
Diewald L, Rupp J, Dreger M, Hucho F, Gillen C, Nawrath H (2002) Activation by acidic pH of CLC-7 expressed in oocytes from Xenopus laevis. Biochem Biophys Res Commun 291:421–424
Ding D, Moskowitz SI, Li R, Lee SB, Esteban M, Tomaselli K, Chan J, Bergold PJ (2000) Acidosis induces necrosis and apoptosis of cultured hippocampal neurons. Exp Neurol 162:1–12
Eladari D, Blanchard A, Leviel F, Paillard M, Stuart-Tilley AK, Alper SL, Podevin RA (1998) Functional and molecular characterization of luminal and basolateral \({{\text{Cl}}^{ - } } \mathord{\left/ {\vphantom {{{\text{Cl}}^{ - } } {{\text{HCO}}^{ - }_{{\text{3}}} }}} \right. \kern-\nulldelimiterspace} {{\text{HCO}}^{ - }_{{\text{3}}} }\) exchangers of rat thick limbs. Am J Physiol Renal Physiol 275:F334–F342
Estevez R, Boettger T, Stein V, Birkenhager R, Otto E, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl− channel β-subunit crucial for renal Cl− reabsorption and inner ear K+ secretion. Nature 414:558–561
Fan H-T, Morishima S, Kida H, Okada Y (2001) Phloretin differentially inhibits volume-sensitive and cAMP-activated, but not Ca-activated, Cl− channels. Br J Pharmacol 133:1096–1106
Friedrich T, Breiderhoff T, Jentsch TJ (1999) Mutational analysis demonstrates that ClC-4 and ClC-5 directly mediate plasma membrane currents. J Biol Chem 274:896–902
Goldman SA, Pulsinelli WA, Clarke WY, Kraig RP, Plum F (1989) The effects of extracellular acidosis on neurons and glia in vitro. J Cereb Blood Flow Metab 9:471–477
Helbig H, Korbmacher C, Kuhner D, Berweck S, Wiederholt M (1988) Characterization of \({{\text{Cl}}^{ - } } \mathord{\left/ {\vphantom {{{\text{Cl}}^{ - } } {{\text{HCO}}^{ - }_{{\text{3}}} }}} \right. \kern-\nulldelimiterspace} {{\text{HCO}}^{ - }_{{\text{3}}} }\) exchange in cultured bovine pigmented ciliary epithelium. Exp Eye Res 47:515–523
Hélix N, Strobaek D, Dahl BH, Christophersen P (2003) Inhibition of the endogenous volume-regulated anion channel (VRAC) in HEK293 cells by acidic di-aryl-ureas. J Membr Biol 196:83–94
Iyer R, Iverson TM, Accardi A, Miller C (2002) A biological role for prokaryotic ClC chloride channels. Nature 419:715–718
Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568
Jordt SE, Jentsch TJ (1997) Molecular dissection of gating in the ClC-2 chloride channel. EMBO J 16:1582–1592
Kawasaki M, Fukuma T, Yamauchi K, Sakamoto H, Marumo F, Sasaki S (1999) Identification of an acid-activated Cl− channel from human skeletal muscles. Am J Physiol Cell Physiol 277:C948–C954
Kim KH, Shcheynikov N, Wang Y, Muallem S (2005) SLC26A7 is a Cl− channel regulated by intracellular pH. J Biol Chem 280:6463–6470
Lambert S, Oberwinkler J (2005) Characterization of a proton-activated, outwardly rectifying anion channel. J Physiol 567:191–213
Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C (1998) Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: a perspective on the contributions of apoptosis and necrosis. Brain Res Bull 46:281–309
Mo L, Hellmich HL, Fong P, Wood T, Embesi J, Wills NK (1999) Comparison of amphibian and human ClC-5: similarity of functional properties and inhibition by external pH. J Membr Biol 168:253–264
Mori S, Morishima S, Takasaki M, Okada Y (2002) Impaired activity of volume-sensitive anion channel during lactacidosis-induced swelling in neuronally differentiated NG108-15 cells. Brain Res 957:1–11
Nabekura T, Morishima S, Cover TL, Mori S, Kannan H, Komune S, Okada Y (2003) Recovery from lactacidosis-induced glial cell swelling with the aid of exogenous anion channels. Glia 41:247–259
Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA (1991) Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol Regul Integr Comp Physiol 260:R581–R588
Nilius B, Droogmans G (2003) Amazing chloride channels: an overview. Acta Physiol Scand 177:119–147
Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Nilius B, Oike M, Zahradnik I, Droogmans G (1994) Activation of a Cl− current by hypotonic volume increase in human endothelial cells. J Gen Physiol 103:787–805
Nobles M, Higgins CF, Sardini A (2004) Extracellular acidification elicits a chloride current that shares characteristics with ICl(swell). Am J Physiol Cell Physiol 287:C1426–C1435
Oiki S, Kubo M, Okada Y (1994) Mg2+ and ATP-dependence of volume-sensitive Cl− channels in human epithelial cells. Jpn J Physiol 44:S77–S79
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol 273:C755–C789
Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S (2001) Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532:3–16
Okada Y, Maeno E, Shimizu T, Manabe K, Mori S, Nabekura T (2004) Dual roles of plasmalemmal chloride channels in induction of cell death. Pflügers Arch 448:287–295
Rehncrona S (1985) Brain acidosis. Ann Emerg Med 14:770–776
Sabirov RZ, Okada Y (2004) ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn J Physiol 54:7–14
Sabirov RZ, Prenen J, Droogmans G, Nilius B (2000) Extra- and intracellular proton-binding sites of volume-regulated anion channels. J Membr Biol 177:13–22
Sauve R, Cai S, Garneau L, Klein H, Parent L (2000) pH and external Ca2+ regulation of a small conductance Cl− channel in kidney distal tubule. Biochim Biophys Acta 1509:73–85
Shen MR, Wu SN, Chou CY (1996) Volume-sensitive chloride channels in the primary culture cells of human cervical carcinoma. Biochim Biophys Acta 1315:138–144
Siesjo BK (1988) Acidosis and ischemic brain damage. Neurochem Pathol 9:31–88
Siesjo BK (1993) Basic mechanisms of traumatic brain damage. Ann Emerg Med 22:959–969
Siesjo BK, Katsura K, Kristian T (1996) Acidosis-related damage. Adv Neurol 71:209–233
Strange K, Emma F, Jackson PS (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol Cell Physiol 270:C711–C730
Tomlinson FH, Anderson RE, Meyer FB (1993) Brain pHi, cerebral blood flow, and NADH fluorescence during severe incomplete global ischemia in rabbits. Stroke 24:435–443
Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118:687–698
Xiong ZG, Chu XP, Simon RP (2006) Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol 209:59–68
Xu L, Glassford AJ, Giaccia AJ, Giffard RG (1998) Acidosis reduces neuronal apoptosis. Neuroreport 9:875–879
Yamamoto S, Ehara T (2006) Acidic extracellular pH-activated outwardly rectifying chloride current in mammalian cardiac myocytes. Am J Physiol Heart Circ Physiol 290:H1905–H1914
Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ (2004) Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 101:6752–6757
Acknowledgments
The authors thank E.L. Lee for reading the manuscript, K. Shigemoto and M. Ohara for technical assistance, and T. Okayasu for secretarial assistance. This work was supported by Grants-in-Aid for Scientific Research from MEXT and JSPS and by funds from Japan China Medical Association.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai-Yan Wang and Takahiro Shimizu equally contributed to this study.
Rights and permissions
About this article
Cite this article
Wang, HY., Shimizu, T., Numata, T. et al. Role of acid-sensitive outwardly rectifying anion channels in acidosis-induced cell death in human epithelial cells. Pflugers Arch - Eur J Physiol 454, 223–233 (2007). https://doi.org/10.1007/s00424-006-0193-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0193-z