Abstract
The Golgi complex undergoes considerable structural remodeling during differentiation of urothelial cells in vivo and in vitro. It is known that in a healthy bladder the differentiation from the basal to the superficial cell layer leads to the formation of the tightest barrier in our body, i.e., the blood–urine barrier. In this process, urothelial cells start expressing tight junctional proteins, apical membrane lipids, surface glycans, and integral membrane proteins, the uroplakins (UPs). The latter are the most abundant membrane proteins in the apical plasma membrane of differentiated superficial urothelial cells (UCs) and, in addition to well-developed tight junctions, contribute to the permeability barrier by their structural organization and by hindering endocytosis from the apical plasma membrane. By studying the transport of UPs, we were able to demonstrate their differentiation-dependent effect on the Golgi architecture. Although fragmentation of the Golgi complex is known to be associated with mitosis and apoptosis, we found that the process of Golgi fragmentation is required for delivery of certain specific urothelial differentiation cargoes to the plasma membrane as well as for cell–cell communication. In this review, we will discuss the currently known contribution of the Golgi complex to the formation of the blood–urine barrier in normal UCs and how it may be involved in the loss of the blood–urine barrier in cancer. Some open questions related to the Golgi complex in the urothelium will be highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The formation of an effective blood–urine barrier, which is the tightest epithelial barrier in the body, is of fundamental importance for bladder function and metabolic homeostasis of the terrestrial mammalian species (Hicks 1975). This barrier would not be possible without the specific molecular and morphological adaptations of the urothelium, which lines the renal pelvis, ureters, urinary bladder, and proximal urethra (Romih et al. 2005). The urothelium has two possible permeation pathways (Lasič et al. 2015). The transcellular pathway consists of the apical and basolateral plasma membranes. The main transcellular permeability barrier is the apical plasma membrane of superficial urothelial cells (UCs), which is characterized by numerous specialized features, such as its superficial glycosaminoglycan layer (Parsons 2007), its particular lipid composition (Grasso and Calderón 2009; Resnik et al. 2015) and foremost urothelial plaques composed of the transmembrane proteins uroplakins (UPs) (Yu et al. 1990; Wu et al. 1990; Hu et al. 2002, 2000; Kachar et al. 1999), all of which influence the course of passive diffusion, active transport, and endocytosis. The paracellular pathway consists of the intercellular space and tight junctions, which are extremely impermeable to molecular and ionic flux and are the main barrier to paracellular transport. The barrier between urine and blood has been shown to have the highest recorded transepithelial resistance of all epithelia, with a value of up to 78,000 Ωcm2 (Lewis and Diamond 1976), and the urothelium of the urinary bladder is consistently the least permeable epithelium among most organisms (Lasič et al. 2015).
The urothelium consists of a single layer of small basal urothelial cells (UCs), one to several layers of intermediate UCs, and a layer of highly differentiated superficial UCs, also known as umbrella cells (Fig. 1a). The latter are densely packed with cytoplasmic organelles, including Golgi apparatus and associated vesicles (Jost et al. 1989). Differentiation of superficial UCs follows the expression sequence of tight junction proteins (Varley et al. 2006; Smith et al. 2015; Kreft et al. 2005; Višnjar and Kreft 2013), surface glycans (Zupančič et al. 2014), apical membrane lipids, and the most abundant transmembrane proteins UPs in the apical plasma membrane of UCs (Fig. 1a) (Wu et al. 1994; Yu et al. 1994). UPs form urothelial plaques in the apical plasma membrane facing the lumen of the urinary bladder (12-nm-thick membrane), which are separated by hinges (5 to 7-nm-thick membrane) (Wu et al. 1994). Besides well-developed tight junctions, the UPs contribute to the permeability barrier through their structural organization (Lobban et al. 1998; Kachar et al. 1999; Liang et al. 2001; Min et al. 2003) (Liang et al. 2001; Kachar et al. 1999; Min et al. 2003; Lobban et al. 1998), including through the specific composition and position of a tyrosine-based motif in cytoplasmic domains of UPIa (YTML) and UPIIIa (YTSV) (Kreft et al. 2009a), and by hindering constitutive endocytosis from the apical plasma membrane (Kreft et al. 2009a; Tratnjek et al. 2017). Although UPs have been studied primarily in the mammalian urothelium, they may actually serve conserved and ancient functions in oocyte fertilization and only later acquired their ability to form two-dimensional crystals of 16-nm particles (urothelial plaques) during mammalian divergence, to perform additional functions, including the expansion of superficial UCs and the formation of a highly efficient permeability and mechanical barrier to protect and functionally modify the apical surface of the modern mammalian urothelium (Garcia-Espana et al. 2006; Desalle et al. 2014; Liao et al. 2018).
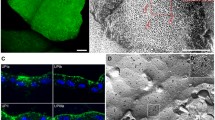
Normal porcine urothelium in vitro and mouse urothelium in vivo and the organization of the Golgi complex in urothelial cells. a Normal porcine UCs grown on a scaffold of human amniotic membrane and labeled with antibodies against uroplakins. The strongest labeling of differentiation-associated markers uroplakins (brown) is seen in the superficial UCs (umbrella cells). b Distribution of GRASP 55 (green) in mouse urothelium. c Co-localization of GRASP 55 (green) and giantin (red) in mouse urothelium. d Perinuclear distribution of GRASP 55 in intermediate UCs. e GRASP 55 distribution over the entire cytoplasm in the umbrella cell. Note: a Immunohistochemistry on formalin-fixed paraffin section. b, c Immunofluorescence on cryo-semithin sections (thickness 300 nm) prepared using the Tokuyasu technique. d, e Immunofluorescence on optical section of the UCs in the direction of the lumen—perpendicular to the apical surface. Scale bars, 50 µm (a, b); 10 µm (c, d, e)
The Golgi complex is tightly integrated into the urothelial cellular system, where, like in other eukaryotic cells, it plays vital functions in the processing and sorting of proteins and lipids and serves as a signaling and a microtubule-organizing center. Here we will review the currently known contribution of the Golgi complex to the formation of the blood–urine barrier, mainly through its association with UP traffic in normal urothelial cells and its possible involvement in the loss of the blood–urine barrier in cancer cells. We will discuss a number of open questions about the structure and function of the Golgi complex in urothelial cell biology.
Dynamic nature of the Golgi complex
As a central hub in the biosynthetic and endocytic pathways that define the urothelial permeability barrier, the Golgi complex constantly receives the flow of cargos and serves as a major processing station in the cell. Due to its dynamic nature, a sophisticated and constantly remodeling mechanism must be established to maintain the architecture and function of the Golgi complex for the uninterrupted transport of proteins and lipids.
In eukaryotic cells, the organization of the Golgi complex varies, although the proteins involved in the formation of the typical Golgi structure are similar. These proteins seem to differ only in the number of their isoforms. Therefore, the consensus is that different forms of the Golgi complex have formed from a single ancestor during evolution (Mowbrey and Dacks 2009), and thus all Golgi complex forms are the result of the different expression of the Golgi proteins (reviewed in (Mironov et al. 2017)).
There are three main morphological forms of the Golgi complex: (1) tubular networks (e.g., in microsporidia); (2) isolated perforated disks (e.g., in Saccharomyces cerevisiae); and (3) partially perforated disks organized in stacks (e.g., in other cells). The number of disks in a stack is in average less than eight (reviewed in Table 2 in the reference (Mironov et al. 2017)). Dozens, or even hundreds, of Golgi stacks in mammalian cells are interconnected into a ribbon-like structure that can act as a single organelle, with alternating compact (i.e., stacked cisternae) and non-compact (i.e., tubular–reticular) sections (Mironov and Beznoussenko 2011). Abundant evidence has been accumulated that a well-organized Golgi complex structure is required for its proper functions (Zhang 2021). However, the ribbon can undergo distinct disassembly processes that reflect the cellular state or extracellular/environmental signals and stress. The most dramatic Golgi complex change occurs in mitosis, when the ribbon is disassembled into vesicles and small non-polarized ministack (Mironov and Beznoussenko 2011) by a combination of unbundling of the ribbon, disk/cisternal unstacking, and vesiculation. In addition, the ribbon can also be fragmented to varying degrees during the progression of disease or cell death, or it may be detached/fragmented and positioned at specific cellular sites to provide additional functions during differentiation (Wei and Seemann 2017).
Such differentiation-dependent Golgi remodeling is observed also in UCs. The localization of Golgi-related markers [GM130 (cis-Golgi matrix protein of 130 kDa), GS15 (Golgi Snare 15 kDa), GS28, Golgin 97, GRASP55 and giantin], shows that in non-differentiated, UP-negative UCs the Golgi complex is mostly organized as a single ribbon-like structure close to the nucleus, whereas in differentiated, UP-positive UCs the Golgi complex is fragmented and spread almost through the entire cell (Fig. 1b–e). This phenomenon is observed in in vitro as well in vivo models. The role of such remodeling the Golgi complex in the biology of UCs and the formation of the blood–urine barrier will be discussed in the next sections.
Golgi complex remodeling enables delivery of specific urothelial differentiation cargoes to the plasma membrane and the formation of the blood–urine barrier
The involvement of the Golgi complex in the modification of UPs
Although the urothelium is a multifunctional epithelium also involved in sensory transduction that enables bladder fullness sensation and proper micturition (Khandelwal et al. 2009; Winder et al. 2014; Marshall et al. 2020; Dalghi et al. 2021), its maintenance of the blood–urine barrier is its most crucial function. That Golgi complex is the site of assembly of the thick luminal, i.e., apical plasma membrane of the superficial UCs, which makes a key contribution to the blood–urine barrier, was already suggested by Raymond Marian Hicks (Hicks 1966), who found similar thick patches of membranes (then termed asymmetric unit membrane, AUM) in the walls of the Golgi cisternae and in the apical plasma membrane. This implied the membrane flow from the Golgi complex to the cell surface. This and subsequent studies proposed that the membrane is transported to the luminal surface in the form of round- to discoid-shaped vesicles, derived from the Golgi cisternae, which fuse with, and become part of the apical plasma membrane (Severs and Hicks 1979; Alroy et al. 1982; Koss 1969; Hicks 1966). This hypothesis was later tested checked in several studies, which demonstrated that UPs are synthesized in the endoplasmic reticulum (ER) where they form two types of heterodimers (UPIa/UPII and UPIb/UPIIIa) before they exit the ER (Tu et al. 2002). UP-heterodimers are then transported from the ER to the Golgi complex (Višnjar et al. 2017), where Golgi-associated glycosylation reactions may exhibit UP-changes. Although GA-associated glycosylation reactions are clearly demonstrated in different cell types and animal species (Roth 1996), this process is poorly known for Golgi complex in UCs. It is identified that UPIb isolated from mouse and human urothelial plaques, and UPIIIa isolated from mouse, cattle, and human urothelial plaques contain complex glycans (Xie et al. 2006; Malagolini et al. 2000). The involvement of Golgi complex in the modification of UPs is also supported by the observation that the prosequence of UPII can be cleaved by the Golgi-protease furin (Hu et al. 2008). However, understanding the relationship between Golgi-associated glycosylation and Golgi structure is still enigmatic. Nevertheless, insights into the mechanisms of Golgi complex biogenesis from ribbon-like to fragmented form in UCs have been revealed in the last decade.
The Golgi complex biogenesis from the ribbon-like to fragmented form
The functional reorganization of the Golgi complex is accompanied by specific rearrangements of the microtubules and intermediate filaments, which coincide with the differentiation of the UCs. The differentiation-dependent fragmentation of the Golgi complex and the subsequent spreading of Golgi to the cell periphery represent one of the key events that promote the uniform delivery of UPs throughout the apical plasma membrane of differentiating UCs and is thus of great importance for the final proper formation and maintenance of the blood–urine barrier (Kreft et al. 2010a). In FRAP experiments, the prominent recovery of fluorescently marked GalT into the bleached area was observed in UCs with ribbon-like Golgi, indicating that GalT could exchange between Golgi stacks within the ribbon-like Golgi. In UCs with transitional or fragmented Golgi, weak (i.e., approximately one order of magnitude slower in fragmented Golgi compared with ribbon-like Golgi) or no FRAP fluorescence into the Golgi region was found. Thus, in differentiated UCs, FRAP of GalT–GFP is inhibited, which suggests that Golgi fragments are functionally separated (Kreft et al. 2010a). Whether this applies to all Golgi proteins or only to GalT-GFP and whether the condition is the same in vivo, is currently not yet known.
Importantly, during such Golgi complex remodeling, it remains functional. A generation of specific UP cDNA constructs made it possible to study the dynamics of UPIb/UPIIIa transport in living UCs. Using cell transfection, time-lapse microscopy, immunohistochemistry and freeze-fracture replica immunolabeling, the biosynthesis and transport of UPs were confirmed in UCs and also in those cells that do not endogenously synthesize UPs and primarily have a ribbon-like Golgi complex (Višnjar et al. 2017). This study demonstrated the direct effect of UPs expression on Golgi complex fragmentation, allowing Golgi-outposts to be distributed as close as possible to the sites of cargo delivery at the plasma membrane (Višnjar et al. 2017). In post-Golgi compartments the 16-nm UP particles composed of six heterotetramers of UPIa/UPII-UPIb/UPIIIa (Kachar et al. 1999) gradually arrange into semi-crystalline urothelial plaques (Hudoklin et al. 2011, 2012; Warren and Hicks 1970; Severs and Hicks 1979). Freeze-fracture images revealed post-Golgi compartments, namely UP-positive discoidal or fusiform-shaped vesicles (DFVs) in close association with the Golgi and apical plasma membrane. Since the size of urothelial plaques in the membrane of DFVs resembles those found in close proximity to larger plaques in the apical plasma membrane, these associations are thought to be ideally configured to function in intracellular synthesis and transport as well as cytoplasmic-plasmalemmal transfer and also the continuous progressive incorporation of UP particles into urothelial plaques in the apical plasma membrane (Kreft and Robenek 2012).
Transport of urothelial cargoes from the Golgi complex to the plasma membrane
Analysis of the Golgi-derived structures that transport UPs to the plasma membrane revealed that neither COPI nor COPII nor clathrin are present on their surface (Višnjar et al. 2017). Presumably, COPI-dependent vesicles are too small for the transport of large cargo aggregates including proteinaceous membrane thickenings in UCs (Mironov et al. 2017). This suggests that UP-containing structures likely utilize post-Golgi trafficking mechanisms used by a number of constitutively secreted cargo proteins (Polishchuk et al. 2003; Luini et al. 2008) and was indicated in the in vivo studies (Hudoklin et al. 2011, 2012). One of the common features of these mechanisms is the movement of post-Golgi carriers along microtubules and actin filaments. We have demonstrated the co-localization of microtubules and actin filaments with UPIb/UPIIIa-EGFP-positive vesicles and confirmed that UPIb/UPIIIa-EGFP transport depends on microtubules and actin filaments by determining their velocity and using depolymerization approaches. UP-trafficking mechanism currently proposed for still differentiating UCs is the following: UPIb/UPIIIa-EGFP-positive vesicles initially move from the Golgi complex to the plasma membrane along microtubules that enable transport at higher velocity. However, closer to the plasma membrane, UPIb/UPIIIa-EGFP-positive vesicles transition to actin filaments, which enable lower transport velocity and thus prime UPIb/UPIIIa-EGFP-positive vesicles for targeting to the plasma membrane (Višnjar et al. 2017).
When UCs become terminally differentiated with the bulk of UPs already at the apical plasma membrane, the microtubules, actin filaments and intermediate filaments are reorganized (Tratnjek et al. 2017; Kreft et al. 2009b, 2005, 2010a; Romih et al. 2002, 1999). Microtubular organization in the basal, central and subapical cytoplasm of highly differentiated UCs is diminished and actin filaments are mainly removed from the subapical cytoplasm (Tratnjek et al. 2017). This probably also contributes to the impeded endocytotic events. In contrast, trajectorial cytokeratin network accumulate in the apical cytoplasm (Kreft et al. 2002; Veranic and Jezernik 2002; Veranic et al. 2004), probably preventing the microtubular-dependent centralization of Golgi and maintaining the peripheral fragmentation of the Golgi complex in differentiated UCs. Currently, it is still not known, how the UP-trafficking machinery drives Golgi fragmentation in UCs and which transcriptional program and epigenetic changes are required for its reorganization.
In addition to UPs, the urothelium also has numerous surface receptors and ion channels that affect membrane conductance and are also considered in the context of maintaining the blood–urine barrier. The apical plasma membrane of the urothelium contains sodium, potassium, and calcium channels, as well as other cation channels. The basolateral plasma membrane of the urothelium also contains sodium and potassium channels as well as chloride channels, Na + /H + and Cl//HCO3 exchangers, and ATPase pumps (reviewed in (Lasič et al. 2015; Dalghi et al. 2020). Along with mechanosensitive ion channels, the urothelium contains many other receptors, e.g., the transient receptor potential (TRP) cation channels, which may play a role in the mechano- and/or chemosensory function of bladder urothelium (Winder et al. 2014). More specifically, TRPV4 plays a role in the transduction of intravesical mechanical pressure and thus bladder function (Gevaert et al. 2007). This channel as well as other TRP channels are gaining attention as possible new drug targets for the treatment of different urinary tract and bladder pathophysiologies (Lasič et al. 2015). Apart from the TRP channels, the urothelium receives many inputs through a vast number of other surface receptors and ion channels such as nicotinic and muscarinic receptors, the purinergic P2X family of ATP receptors, TRAAK and TREK-1 channels, acid-sensitive ion channels (Wang et al. 2005; Khandelwal et al. 2009; Araki et al. 2008; Birder and Andersson 2013), and the mechanosensitive ion channel PIEZO2, which it is required for low-threshold bladder-stretch sensing and urethral micturition reflexes (Marshall et al. 2020). However, the transport of these surface receptors and ion channels through the Golgi complex to the plasma membrane of UCs and their exact ultrastructural localization in the plaque or hinge regions of UCs is not known. The possible role of the Golgi complex in their formation and perhaps even their reciprocal effect on the Golgi complex itself have also not been explored. The relationship between the Golgi complex and the tight junctional proteins has also not yet been investigated.
Fragmentation of the Golgi complex enables long distance urothelial cell–cell communication
Communication between cells is crucial for unicellular organisms, especially when they are forming a multicellular structure, like a biofilm, and for all multicellular organisms (Dubey and Ben-Yehuda 2011). Tunneling membrane nanotubes (TNTs) are membrane protrusions connecting nearby or distant cells in vitro and in vivo. Since their discovery in 2004, TNTs have gained much attention as critical players in intercellular communication (Rustom et al. 2004). They mediate intercellular exchange of cargoes including proteins, RNAs, miRNAs, ions, bacteria, viruses, prions, and organelles between connected cells (Vignais et al. 2017).
TNTs transport organelles such as lysosomes, mitochondria, the endoplasmic reticulum and Golgi complex (Antanavičiūtė et al. 2014; Zhang et al. 2021; Wang et al. 2011). Recently, it has been shown that the location of the centrosome not only predicts the site of TNT formation but can also facilitate intercellular transport by regulating microtubule formation and Golgi orientation (Dubois et al. 2021). We have found that Golgi outposts are also seen in normal and cancerous UCs (Resnik et al., in submission), suggesting Golgi fragmentation in the context of urothelial cell–cell communication.
Since TNTs are very sensitive to mechanical stress and chemical fixation, many basic properties of TNTs are still poorly known. Correlative phase contrast and fluorescence microscopy have shown that less than 30% of TNTs present in living cells are retained after the immunolabeling procedure (our unpublished data). Nevertheless, the mechanism and nature of Golgi fragmentation in TNT remain to be explored.
Contribution of the Golgi complex to the loss of the blood–urine barrier in cancer
Bladder cancer is seventh most common cancer in men. Its epidemiology still shows a clear male predominance, and incidence and mortality rates differ per European country, probably due to differences in risk factors, detection, and availability of treatments (Witjes et al. 2021). On the global level, incidence and mortality rates vary due to different methodologies and diagnostic practices. Approximately 25% of patients with bladder cancer present with muscle-invasive or metastatic disease, others have non-muscle invasive bladder cancer (NMIBC) (Taskovska et al. 2020).
Even the first, epoch-making studies of the bladder epithelium using light and electron microscopy and histochemistry showed that the bladder is excellently adapted to urine and its contents. On the other hand, the urothelium bears the brunt of the attack by pollutants and various oncogenes. In 1978, Melicow assumed that the ongoing battle may be of long duration or recurrent and influence on specialized UC apical plasma membrane and the Golgi complex (Melicow 1978). In this chapter, we discuss the possible indirect role of the Golgi complex in the increased transcellular permeability of the cancerous urothelium. We will not refer here to the loss of the blood–urine barrier due to the loss of the tight junctions and the increased paracellular permeability, which is one of the first steps that could lead to the invasion of cancer cells into the blood vessels (Jerman et al. 2021).
It is known that the luminal membrane is changed in cancer UCs and, instead of urothelial plaques, a thinner, smooth, flexible membrane develops with a filamentous glycocalyx above it (Melicow 1978; Resnik et al. 2015; Yu et al. 2016; Zupančič et al. 2014, 2011; Lobban et al. 1998; Olsburgh et al. 2003). Remarkably, altered glycosylation has been a hallmark of most cancer cells (Zhang 2021). To understand the causes of altered Golgi structure and function in bladder cancer, efforts must be made to characterize the structural proteins of the Golgi under physiological and pathological conditions.
When we transfected HeLa cells with UPIb-EGFP or UPIb/UPIIIa, which do not endogenously express UPs, the Golgi complex was fragmented (Višnjar et al. 2017). During urothelial carcinogenesis, UCs lose expression of UPs (Lobban et al. 1998; Zupancic and Romih 2013) and fragmentation of the Golgi complex in muscle-invasive bladder cancer cells T24 is not achieved (our unpublished data). This differs from descriptions in other cell types and disease states. It is known that a fragmented Golgi ribbon is commonly associated with many stress and pathological conditions, including apoptosis (Machamer 2015; Lane et al. 2002), bacterial or viral infections (Jimenez et al. 2016; Lavi et al. 1996), Alzheimer’s disease (Joshi et al. 2014, 2015), Parkinson’s disease (Rendón et al. 2013; Tomás et al. 2021) and various forms of cancer (Núñez-Olvera et al. 2020; Petrosyan 2015). Despite similar phenotypic characteristics among these diseases, the mechanisms that cause Golgi fragmentation and dysfunction can range from imbalanced membrane flux, altered microtubule dynamics, to post-translational modifications or irreversible proteolytic cleavage of Golgi structural proteins. It is not clear whether the mechanisms that drive Golgi ribbon disassembly in mitosis or during differentiation are also underpinning Golgi fragmentation during disease progression. In fact, the correlation between the observed morphological alterations and dysfunction of the Golgi complex is often unclear, as the fragmentation may directly cause, partially contribute to, or merely be the outcome of pathology (Wei and Seemann 2017).
Golgi phosphoprotein 3 (GOLPH3) is a highly conserved peripheral membrane protein of ~ 34 kDa that is localized in the trans-Golgi network and dynamically exchanges with a large cytosolic pool (Wu et al. 2000; Kuna and Field 2019; Cavieres et al. 2020). GOLPH3 plays a role in anterograde and retrograde Golgi trafficking and is considered as a hub for regulating the Golgi complex organization and function. It is also considered as the first oncoprotein of the Golgi complex to play an important role in various types of cancer (Yu et al. 2020), including bladder cancer (Zhang et al. 2015). Overexpression of GOLPH3 had significant correlation with poorer survival for bladder cancer patients. The action of GOLPH3 probably occurs via several mechanisms, including activation of PI3K/AKT/mTOR pathway (Zhang et al. 2015). Its inhibition in cells overexpressing GOLPH3 could therefore be a therapeutic approach in cancer.
The association of GOLPH3 with Golgi membranes depends on its binding to phosphatidylinositol-4-phosphate (PtdIns4P), which promotes the exit of vesicles in the Golgi for transport to the plasma membrane. PtdIns4P is enriched in the trans-Golgi and therefore recruits GOLPH3. This allows the formation of a GOLPH3 complex, which is formed when GOLPH3 binds to myosin18A, which in turn binds F-actin. This complex generates a pulling force to extract vesicles from the Golgi; interference with this GOLPH3 complex leads to a drastic reduction in vesicle trafficking (Kuna and Field 2019). Since GOLPH3 plays a role in anterograde and retrograde Golgi trafficking and is involved in vesicle budding from the trans-Golgi and in several post-Golgi protein trafficking events, it may also be involved in UP trafficking to the plasma membrane of UCs. However, this mechanism has not yet been explored in UCs. In conclusion, knowledge of the molecular mechanisms involved in alterations to the urothelial Golgi complex phenotype under pathological conditions is limited but urgently needed to decipher the pathogenesis of various urinary tract diseases and to develop new therapeutic approaches.
Summary and open questions
Many of the pathways to the apical and basolateral UC surfaces involve transient passage through the Golgi complex. While recent studies significantly advanced our understanding of how the Golgi complex is involved in UP-transport to the plasma membrane and as such is critical for the formation and maintenance of the blood–urine barrier, it is still not known how the UP-transport machinery drives Golgi-fragmentation in UCs and what transcriptional programs and epigenetic changes are required for the Golgi complex reorganization. Moreover, it looks like there are different types of Golgi fragmentation in UCs, depending on what cell cycle phase the cell is in and what stage of differentiation it is in. If we look at the morphology of the Golgi complex in highly differentiated superficial UCs (umbrella cells) (Fig. 1e and Fig. 2a, b, d), the Golgi complex is still very active, so its fragmentation is not the same as in mitosis.
Representative examples of the Golgi complexes from umbrella cells. a Low magnification of the umbrella cell. The green box indicates the Golgi complex. The red box shows the ER exit site. b Enlargement of the area inside green box in (a). c Enlargement of the area inside the red box in (a). d Immuno EM based on cryosections according to Tokuyasu. Labeling for GRASP55 is present over Golgi cisternae. Scale bars 600 nm (a); 220 nm (b); 180 nm (c); 90 nm (d)
There are several additional unclear problems. It is not clear how the UPs are transported from the ER to the Golgi complex. In umbrella cells, there are ER exit sites (ERES) (Fig. 2c and Fig. 3c, d, f). However, the sections of these compartments are not round and the round profiles which usually are observed within ERES are not seen. Part of their limiting membrane appears rather plane. These membranes are similar to membranes of the FVs, although these membranes were not coated with glycocalyx similar to that observed on the luminal surface of the plaque membranes of FVs and the apical plasma membrane (Fig. 3e, g, h). This may be the result of not yet formed and/or non-aggregated UP particles along with non-glycosylation. The urothelial plaques are covered by glycocalyx. There is less glycocalyx in the urothelium of mice than in the urothelium of humans and pigs (please see Fig. 3 in the reference (Kreft et al. 2010b) and Fig. 6 in the reference (Jerman et al. 2014)). This difference could be due to physiological and structural differences between the species. It could be that the glycocalyx is intensively formed as the first line of defense against urine permeability, which is later achieved and maintained in the animals with more concentrated urine by a high density of UP particles in superficial urothelial cells. The UP particles contribute to the apical plasma membrane region (also called urothelial plaque or asymmetric unit membrane (AUM)) being thicker than in the hinge region (see Fig. 2B in the reference (Hudoklin et al. 2012)). It is believed that the thicker E-half of the apical plasma membrane is mainly due to the specific structure of UPs with the bulk of extracellular part. Nevertheless, the presence of ERES in the umbrella cells suggests that there occurs intensive synthesis of membranes and that these membranes are not necessary for mitotic division but for the generation of FVs.
Structure of the Golgi complex and its derivatives in umbrella cells. a The Golgi complex with the presumably COPI-dependent vesicles only at the Golgi cis–side. b Ministack with COPI vesicles at all levels. c, d ER exit sites (yellow arrows in c; green box in d). e Clathrin-coated bud on the FV. f Enlargement of the area inside green box in (d). The yellow arrow shows COPII-coated bud. Deformation of round profiles typical for other cell types. g Enlargement of the area inside the blue box in (e). h Image shows tight attachment of post-Golgi transport vesicle to the FV. i Enlargement of the area inside the red box in (h). The yellow arrow shows attachment of post-Golgi transport vesicle to the FV. Scale bars 220 nm (a, c, d); 110 nm (b); 200 nm (e, h)
In umbrella cells, the Golgi complex contains COPI-vesicles near its rims (Fig. 3a, b). The urothelial plaques consist of two-dimensional (2D) crystals of hexagonally packed 16 nm UP particles (Min et al. 2003). Therefore, aggregates of two such particles are already incompatible in size with COPI-dependent vesicles. Moreover, near-Golgi vesicles are roundish (Fig. 3h, i) in contrast to the round profiles near ERES (Fig. 3d, f). This suggests that COPI vesicles are not involved in the transport of UP particles through the Golgi complex. On the other hand, it is known that COPI vesicles do not participate in the recycling of Golgi resident proteins (Mironov and Beznoussenko 2019; Beznoussenko et al. 2022) and UP particles are rather big for diffusion along the membrane continuum within Golgi cisternae.
During maturation of umbrella cells, the Golgi complex becomes larger. The GM130-positive compartment unites the Golgi stacks and the trans-most cisterna (see Fig. 2 of (Hudoklin et al. 2009)). This situation is similar to the situation in neurons, where the cis-most cisterna joins the Golgi stacks and is the opposite to the situation in fibroblasts, where the trans-most cisterna joins the Golgi stack (Mironov et al. 2017; Beznoussenko et al. 2022). However, it seems that the Golgi complex contains only limited amount of UPs. This suggests that in umbrella cells, there is a stimulation of synthesis of Golgi enzymes and proteins of Golgi matrix.
When UPs were synthesized in MDCK cells, they formed spots which move peripherally. This process is mostly microtubule dependent (Višnjar et al. 2017). Thus, the appearance of UPs induces the interaction of post-Golgi vesicles and microtubules. The mechanisms of this process are unknown. We have found that more glycocalyx is present in these vesicles when UCs are not terminally differentiated, which is usually the case when UCs are grown in vitro (see Fig. 1C, F, K in the reference (Predojević et al. 2022).
Uroplakins are glycosylated and pass through the Golgi complex (see Fig. 3 by (Višnjar et al. 2017). However, further ultrastructural studies are needed to analyze these proteins within the Golgi cisternae. It could also be that UP-positive membranes are present near the Golgi complex being connected with it. Thus, a further 3D analysis of the Golgi complex is highly necessary.
In conclusion, the Golgi complex plays an important role in the UC differentiation, however there is still question what factors are responsible for the differentiation associated interplay between the Golgi complex and UCs. Nevertheless, there is a growing awareness that the Golgi complex plays an important role in precisely tuned blood–urine barrier machinery within the bladder urothelium and may also play a role in a variety of bladder diseases. This therefore needs to be considered in future research and in the development of more effective disease treatment.
Data availability
Authors can confirm that all relevant data are included in the article.
References
Alroy J, Merk FB, Morré DJ, Weinstein RS (1982) Membrane differentiation in the Golgi apparatus of mammalian urinary bladder epithelium. Anat Rec 203(4):429–440. https://doi.org/10.1002/ar.1092030402
Antanavičiūtė I, Rysevaitė K, Liutkevičius V, Marandykina A, Rimkutė L, Sveikatienė R, Uloza V, Skeberdis VA (2014) Long-distance communication between laryngeal carcinoma cells. PLoS One 9(6):e99196. https://doi.org/10.1371/journal.pone.0099196
Araki I, Du S, Kobayashi H, Sawada N, Mochizuki T, Zakoji H, Takeda M (2008) Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol 15(8):681–687. https://doi.org/10.1111/j.1442-2042.2008.02052.x
Beznoussenko GV, Kweon HS, Sesorova IS, Mironov AA (2022) Comparison of the cisterna maturation-progression model with the kiss-and-run model of intra-Golgi transport: role of cisternal pores and cargo domains. Int J Mol Sci. https://doi.org/10.3390/ijms23073590
Birder L, Andersson KE (2013) Urothelial signaling. Physiol Rev 93(2):653–680. https://doi.org/10.1152/physrev.00030.2012
Cavieres VA, Cerda-Troncoso C, Rivera-Dictter A, Castro RI, Luchsinger C, Santibañez N, Burgos PV, Mardones GA (2020) Human Golgi phosphoprotein 3 is an effector of RAB1A and RAB1B. PLoS One 15(8):e0237514. https://doi.org/10.1371/journal.pone.0237514
Dalghi MG, Montalbetti N, Carattino MD, Apodaca G (2020) The urothelium: life in a liquid environment. Physiol Rev 100(4):1621–1705. https://doi.org/10.1152/physrev.00041.2019
Dalghi MG, Ruiz WG, Clayton DR, Montalbetti N, Daugherty SL, Beckel JM, Carattino MD, Apodaca G (2021) Functional roles for PIEZO1 and PIEZO2 in urothelial mechanotransduction and lower urinary tract interoception. JCI Insight. https://doi.org/10.1172/jci.insight.152984
Desalle R, Chicote JU, Sun TT, Garcia-España A (2014) Generation of divergent uroplakin tetraspanins and their partners during vertebrate evolution: identification of novel uroplakins. BMC Evol Biol 14:13. https://doi.org/10.1186/1471-2148-14-13
Dubey GP, Ben-Yehuda S (2011) Intercellular nanotubes mediate bacterial communication. Cell 144(4):590–600. https://doi.org/10.1016/j.cell.2011.01.015
Dubois F, Galas L, Elie N, Le Foll F, Bazille C, Bergot E, Levallet G (2021) Centrosome, the newly identified passenger through tunneling nanotubes, increases binucleation and proliferation marker in receiving cells. Int J Mol Sci 22:9680. https://doi.org/10.3390/ijms22189680
Garcia-Espana A, Chung PJ, Zhao X, Lee A, Pellicer A, Yu J, Sun TT, Desalle R (2006) Origin of the tetraspanin uroplakins and their co-evolution with associated proteins: implications for uroplakin structure and function. Mol Phylogenet Evol 41(2):355–367. https://doi.org/10.1016/j.ympev.2006.04.023
Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van Leuven F, Voets T, De Ridder D, Nilius B (2007) Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117(11):3453–3462. https://doi.org/10.1172/jci31766
Grasso EJ, Calderón RO (2009) Urinary bladder membrane permeability differentially induced by membrane lipid composition. Mol Cell Biochem 330(1–2):163–169. https://doi.org/10.1007/s11010-009-0129-y
Hicks RM (1966) The function of the Golgi complex in transitional epithelium synthesis of the thick cell membrane. J Cell Biol 30(3):623–643. https://doi.org/10.1083/jcb.30.3.623
Hicks RM (1975) The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc 50(2):215–246
Hu P, Deng FM, Liang FX, Hu CM, Auerbach AB, Shapiro E, Wu XR, Kachar B, Sun TT (2000) Ablation of uroplakin III gene results in small urothelial plaques, urothelial leakage, and vesicoureteral reflux. J Cell Biol 151(5):961–972
Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT (2002) Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283(6):F1200-1207. https://doi.org/10.1152/ajprenal.00043.2002
Hu CC, Bachmann T, Zhou G, Liang FX, Ghiso J, Kreibich G, Sun TT (2008) Assembly of a membrane receptor complex: roles of the uroplakin II prosequence in regulating uroplakin bacterial receptor oligomerization. Biochem J 414(2):195–203. https://doi.org/10.1042/bj20080550
Hudoklin S, Zupancic D, Romih R (2009) Maturation of the Golgi apparatus in urothelial cells. Cell Tissue Res 336(3):453–463. https://doi.org/10.1007/s00441-009-0779-9
Hudoklin S, Jezernik K, Neumüller J, Pavelka M, Romih R (2011) Urothelial plaque formation in post-Golgi compartments. PLoS One 6(8):e23636. https://doi.org/10.1371/journal.pone.0023636
Hudoklin S, Jezernik K, Neumüller J, Pavelka M, Romih R (2012) Electron tomography of fusiform vesicles and their organization in urothelial cells. PLoS One 7(3):e32935. https://doi.org/10.1371/journal.pone.0032935
Jerman UD, Veranič P, Kreft ME (2014) Amniotic membrane scaffolds enable the development of tissue-engineered urothelium with molecular and ultrastructural properties comparable to that of native urothelium. Tissue Eng Part C Methods 20(4):317–327. https://doi.org/10.1089/ten.TEC.2013.0298
Jerman UD, Višnjar T, Bratkovič IH, Resnik N, Pavlin M, Veranič P, Kreft ME (2021) Attachment of cancer urothelial cells to the bladder epithelium occurs on uroplakin-negative cells and is mediated by desmosomal and not by classical cadherins. Int J Mol Sci. https://doi.org/10.3390/ijms22115565
Jimenez A, Chen D, Alto NM (2016) How Bacteria subvert animal cell structure and function. Annu Rev Cell Dev Biol 32:373–397. https://doi.org/10.1146/annurev-cellbio-100814-125227
Joshi G, Chi Y, Huang Z, Wang Y (2014) Aβ-induced Golgi fragmentation in Alzheimer’s disease enhances Aβ production. Proc Natl Acad Sci USA 111(13):E1230-1239. https://doi.org/10.1073/pnas.1320192111
Joshi G, Bekier ME 2nd, Wang Y (2015) Golgi fragmentation in Alzheimer’s disease. Front Neurosci 9:340. https://doi.org/10.3389/fnins.2015.00340
Jost SP, Gosling JA, Dixon JS (1989) The morphology of normal human bladder urothelium. J Anat 167:103–115
Kachar B, Liang F, Lins U, Ding M, Wu XR, Stoffler D, Aebi U, Sun TT (1999) Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol 285(2):595–608. https://doi.org/10.1006/jmbi.1998.2304
Khandelwal P, Abraham SN, Apodaca G (2009) Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol 297(6):F1477-1501. https://doi.org/10.1152/ajprenal.00327.2009
Koss LG (1969) The asymmetric unit membranes of the epithelium of the urinary bladder of the rat an electron microscopic study of a mechanism of epithelial maturation and function. Lab Invest 21(2):154–168
Kreft ME, Robenek H (2012) Freeze-fracture replica immunolabelling reveals urothelial plaques in cultured urothelial cells. PLoS One 7(6):e38509. https://doi.org/10.1371/journal.pone.0038509
Kreft ME, Romih R, Sterle M (2002) Antigenic and ultrastructural markers associated with urothelial cytodifferentiation in primary explant outgrowths of mouse bladder. Cell Biol Int 26(1):63–74. https://doi.org/10.1006/cbir.2001.0829
Kreft ME, Sterle M, Veranic P, Jezernik K (2005) Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol 123(4–5):529–539. https://doi.org/10.1007/s00418-005-0770-9
Kreft M, Romih R, Kreft M, Jezernik K (2009a) Endocytotic activity of bladder superficial urothelial cells is inversely related to their differentiation stage. Differentiation 77(1):48–59. https://doi.org/10.1016/j.diff.2008.09.011
Kreft ME, Jezernik K, Kreft M, Romih R (2009b) Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann NY Acad Sci 1152:18–29. https://doi.org/10.1111/j.1749-6632.2008.04004.x
Kreft ME, Di Giandomenico D, Beznoussenko GV, Resnik N, Mironov AA, Jezernik K (2010a) Golgi apparatus fragmentation as a mechanism responsible for uniform delivery of uroplakins to the apical plasma membrane of uroepithelial cells. Biol Cell 102(11):593–607. https://doi.org/10.1042/BC20100024
Kreft ME, Hudoklin S, Jezernik K, Romih R (2010b) Formation and maintenance of blood–urine barrier in urothelium. Protoplasma 246(1–4):3–14. https://doi.org/10.1007/s00709-010-0112-1
Kuna RS, Field SJ (2019) GOLPH3: a Golgi phosphatidylinositol(4)phosphate effector that directs vesicle trafficking and drives cancer. J Lipid Res 60(2):269–275. https://doi.org/10.1194/jlr.R088328
Lane JD, Lucocq J, Pryde J, Barr FA, Woodman PG, Allan VJ, Lowe M (2002) Caspase-mediated cleavage of the stacking protein GRASP65 is required for Golgi fragmentation during apoptosis. J Cell Biol 156(3):495–509. https://doi.org/10.1083/jcb.200110007
Lasič E, Višnjar T, Kreft ME (2015) Properties of the urothelium that establish the blood–urine barrier and their implications for drug delivery. Rev Physiol Biochem Pharmacol 168:1–29. https://doi.org/10.1007/112_2015_22
Lavi E, Wang Q, Weiss SR, Gonatas NK (1996) Syncytia formation induced by coronavirus infection is associated with fragmentation and rearrangement of the Golgi apparatus. Virology 221(2):325–334. https://doi.org/10.1006/viro.1996.0382
Lewis SA, Diamond JM (1976) Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol 28(1):1–40
Liang FX, Riedel I, Deng FM, Zhou G, Xu C, Wu XR, Kong XP, Moll R, Sun TT (2001) Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem J 355(Pt 1):13–18
Liao Y, Chang HC, Liang FX, Chung PJ, Wei Y, Nguyen TP, Zhou G, Talebian S, Krey LC, Deng FM, Wong TW, Chicote JU, Grifo JA, Keefe DL, Shapiro E, Lepor H, Wu XR, DeSalle R, Garcia-España A, Kim SY, Sun TT (2018) Uroplakins play conserved roles in egg fertilization and acquired additional urothelial functions during mammalian divergence. Mol Biol Cell 29(26):3128–3143. https://doi.org/10.1091/mbc.E18-08-0496
Lobban ED, Smith BA, Hall GD, Harnden P, Roberts P, Selby PJ, Trejdosiewicz LK, Southgate J (1998) Uroplakin gene expression by normal and neoplastic human urothelium. Am J Clin Pathol 153(6):1957–1967. https://doi.org/10.1016/s0002-9440(10)65709-4
Luini A, Mironov AA, Polishchuk EV, Polishchuk RS (2008) Morphogenesis of post-Golgi transport carriers. Histochem Cell Biol 129(2):153–161. https://doi.org/10.1007/s00418-007-0365-8
Machamer CE (2015) The Golgi complex in stress and death. Front Neurosci 9:421. https://doi.org/10.3389/fnins.2015.00421
Malagolini N, Cavallone D, Wu XR, Serafini-Cessi F (2000) Terminal glycosylation of bovine uroplakin III, one of the major integral-membrane glycoproteins of mammalian bladder. Biochim Biophys Acta 1475(3):231–237
Marshall KL, Saade D, Ghitani N, Coombs AM, Szczot M, Keller J, Ogata T, Daou I, Stowers LT, Bönnemann CG, Chesler AT, Patapoutian A (2020) PIEZO2 in sensory neurons and urothelial cells coordinates urination. Nature 588(7837):290–295. https://doi.org/10.1038/s41586-020-2830-7
Melicow MM (1978) The urothelium: a battleground for oncogenesis. J Urol 120(1):43–47. https://doi.org/10.1016/s0022-5347(17)57034-2
Min G, Zhou G, Schapira M, Sun TT, Kong XP (2003) Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci 116(Pt 20):4087–4094. https://doi.org/10.1242/jcs.00811
Mironov AA, Beznoussenko GV (2011) Molecular mechanisms responsible for formation of Golgi ribbon. Histol Histopathol 26(1):117–133. https://doi.org/10.14670/hh-26.117
Mironov AA, Beznoussenko GV (2019) Models of intracellular transport: pros and cons. Front Cell Dev Biol 7:146. https://doi.org/10.3389/fcell.2019.00146
Mironov AA, Sesorova IS, Seliverstova EV, Beznoussenko GV (2017) Different Golgi ultrastructure across species and tissues: Implications under functional and pathological conditions, and an attempt at classification. Tissue Cell 49(2Pt A):186–201. https://doi.org/10.1016/j.tice.2016.12.002
Mowbrey K, Dacks JB (2009) Evolution and diversity of the Golgi body. FEBS Lett 583(23):3738–3745. https://doi.org/10.1016/j.febslet.2009.10.025
Núñez-Olvera SI, Chávez-Munguía B, Del Rocío Terrones-Gurrola MC, Marchat LA, Puente-Rivera J, Ruíz-García E, Campos-Parra AD, Vázquez-Calzada C, Lizárraga-Verdugo ER, Ramos-Payán R, Salinas-Vera YM, López-Camarillo C (2020) A novel protective role for microRNA-3135b in Golgi apparatus fragmentation induced by chemotherapy via GOLPH3/AKT1/mTOR axis in colorectal cancer cells. Sci Rep 10(1):10555. https://doi.org/10.1038/s41598-020-67550-0
Olsburgh J, Harnden P, Weeks R, Smith B, Joyce A, Hall G, Poulsom R, Selby P, Southgate J (2003) Uroplakin gene expression in normal human tissues and locally advanced bladder cancer. J Pathol 199(1):41–49. https://doi.org/10.1002/path.1252
Parsons CL (2007) The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology 69(4 Suppl):9–16. https://doi.org/10.1016/j.urology.2006.03.084
Petrosyan A (2015) Onco-Golgi: is fragmentation a gate to cancer progression? Biochem Mol Biol J. https://doi.org/10.21767/2471-8084.100006
Polishchuk EV, Di Pentima A, Luini A, Polishchuk RS (2003) Mechanism of constitutive export from the Golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-Golgi network tubular domains. Mol Biol Cell 14(11):4470–4485. https://doi.org/10.1091/mbc.e03-01-0033
Predojević L, Keše D, Žgur Bertok D, Železnik Ramuta T, Veranič P, Erdani Kreft M, Starčič Erjavec M (2022) A biomimetic porcine urothelial model for assessing Escherichia coli pathogenicity. Microorganisms. https://doi.org/10.3390/microorganisms10040783
Rendón WO, Martínez-Alonso E, Tomás M, Martínez-Martínez N, Martínez-Menárguez JA (2013) Golgi fragmentation is rab and SNARE dependent in cellular models of Parkinson’s disease. Histochem Cell Biol 139(5):671–684. https://doi.org/10.1007/s00418-012-1059-4
Resnik N, Repnik U, Kreft ME, Sepčić K, Maček P, Turk B, Veranič P (2015) Highly selective anti-cancer activity of cholesterol-interacting agents methyl-β-cyclodextrin and ostreolysin A/pleurotolysin B protein complex on urothelial cancer cells. PLoS One 10(9):e0137878. https://doi.org/10.1371/journal.pone.0137878
Romih R, Veranic P, Jezernik K (1999) Actin filaments during terminal differentiation of urothelial cells in the rat urinary bladder. Histochem Cell Biol 112(5):375–380. https://doi.org/10.1007/s004180050419
Romih R, Veranic P, Jezernik K (2002) Appraisal of differentiation markers in urothelial cells. Appl Immunohistochem Mol Morphol 10(4):339–343. https://doi.org/10.1097/00129039-200212000-00009
Romih R, Korosec P, de Mello W Jr., Jezernik K. (2005) Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320 (2):259-268 https://doi.org/10.1007/s00441-004-1005-4
Roth J (1996) Protein glycosylation in the endoplasmic reticulum and the Golgi apparatus and cell type-specificity of cell surface glycoconjugate expression: analysis by the protein A-gold and lectin-gold techniques. Histochem Cell Biol 106(1):79–92. https://doi.org/10.1007/bf02473203
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303(5660):1007–1010. https://doi.org/10.1126/science.1093133
Severs NJ, Hicks RM (1979) Analysis of membrane structure in the transitional epithelium of rat urinary bladder 2 the discoidal vesicles and Golgi apparatus: their role in luminal membrane biogenesis. J Ultrastruct Res 69(2):279–296
Smith NJ, Hinley J, Varley CL, Eardley I, Trejdosiewicz LK, Southgate J (2015) The human urothelial tight junction: claudin 3 and the ZO-1α(+) switch. Bladder (San Franc) 2(1):e9. https://doi.org/10.14440/bladder.2015.33
Taskovska M, Kreft ME, Smrkolj T (2020) Current and innovative approaches in the treatment of non-muscle invasive bladder cancer: the role of transurethral resection of bladder tumor and organoids. Radiol Oncol 54(2):135–143. https://doi.org/10.2478/raon-2020-0025
Tomás M, Martínez-Alonso E, Martínez-Martínez N, Cara-Esteban M, Martínez-Menárguez JA (2021) Fragmentation of the Golgi complex of dopaminergic neurons in human substantia nigra: new cytopathological findings in Parkinson’s disease. Histol Histopathol 36(1):47–60. https://doi.org/10.14670/hh-18-270
Tratnjek L, Romih R, Kreft ME (2017) Differentiation-dependent rearrangements of actin filaments and microtubules hinder apical endocytosis in urothelial cells. Histochem Cell Biol 148(2):143–156. https://doi.org/10.1007/s00418-017-1566-4
Tu L, Sun TT, Kreibich G (2002) Specific heterodimer formation is a prerequisite for uroplakins to exit from the endoplasmic reticulum. Mol Biol Cell 13(12):4221–4230. https://doi.org/10.1091/mbc.E02-04-0211
Varley CL, Garthwaite MA, Cross W, Hinley J, Trejdosiewicz LK, Southgate J (2006) PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol 208(2):407–417. https://doi.org/10.1002/jcp.20676
Veranic P, Jezernik K (2002) Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil Cytoskelet 53(4):317–325. https://doi.org/10.1002/cm.10077
Veranic P, Romih R, Jezernik K (2004) What determines differentiation of urothelial umbrella cells? Eur J Cell Biol 83(1):27–34
Vignais ML, Caicedo A, Brondello JM, Jorgensen C (2017) Cell connections by tunneling nanotubes: effects of mitochondrial trafficking on target cell metabolism, homeostasis, and response to therapy. Stem Cells Int 2017:6917941. https://doi.org/10.1155/2017/6917941
Višnjar T, Kreft ME (2013) Air-liquid and liquid-liquid interfaces influence the formation of the urothelial permeability barrier in vitro. Vitro Cell Dev Biol Anim 49(3):196–204. https://doi.org/10.1007/s11626-013-9585-5
Višnjar T, Chesi G, Iacobacci S, Polishchuk E, Resnik N, Robenek H, Kreft M, Romih R, Polishchuk R, Kreft ME (2017) Uroplakin traffic through the Golgi apparatus induces its fragmentation: new insights from novel in vitro models. Sci Rep 7(1):12842. https://doi.org/10.1038/s41598-017-13103-x
Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G (2005) ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115(9):2412–2422. https://doi.org/10.1172/jci24086
Wang Y, Cui J, Sun X, Zhang Y (2011) Tunneling-nanotube development in astrocytes depends on p53 activation. Cell Death Differ 18(4):732–742. https://doi.org/10.1038/cdd.2010.147
Warren RC, Hicks RM (1970) Structure of the subunits in the thick luminal membrane of rat urinary bladder. Nature 227(5255):280–281. https://doi.org/10.1038/227280b0
Wei JH, Seemann J (2017) Golgi ribbon disassembly during mitosis, differentiation and disease progression. Curr Opin Cell Biol 47:43–51. https://doi.org/10.1016/j.ceb.2017.03.008
Winder M, Tobin G, Zupančič D, Romih R (2014) Signalling molecules in the urothelium. Biomed Res Int 2014:297295. https://doi.org/10.1155/2014/297295
Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, Hernández V, Linares Espinós E, Lorch A, Neuzillet Y, Rouanne M, Thalmann GN, Veskimäe E, Ribal MJ, van der Heijden AG (2021) European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 79(1):82–104. https://doi.org/10.1016/j.eururo.2020.03.055
Wu XR, Manabe M, Yu J, Sun TT (1990) Large scale purification and immunolocalization of bovine uroplakins I, II, and III molecular markers of urothelial differentiation. J Biol Chem 265(31):19170–19179
Wu XR, Lin JH, Walz T, Haner M, Yu J, Aebi U, Sun TT (1994) Mammalian uroplakins a group of highly conserved urothelial differentiation-related membrane proteins. J Biol Chem 269(18):13716–13724
Wu CC, Taylor RS, Lane DR, Ladinsky MS, Weisz JA, Howell KE (2000) GMx33: a novel family of trans-Golgi proteins identified by proteomics. Traffic 1(12):963–975
Xie B, Zhou G, Chan SY, Shapiro E, Kong XP, Wu XR, Sun TT, Costello CE (2006) Distinct glycan structures of uroplakins Ia and Ib: structural basis for the selective binding of FimH adhesin to uroplakin Ia. J Biol Chem 281(21):14644–14653. https://doi.org/10.1074/jbc.M600877200
Yu J, Manabe M, Wu XR, Xu C, Surya B, Sun TT (1990) Uroplakin I: a 27-kD protein associated with the asymmetric unit membrane of mammalian urothelium. J Cell Biol 111(3):1207–1216
Yu J, Lin JH, Wu XR, Sun TT (1994) Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol 125(1):171–182
Yu Y, Skočaj M, Kreft ME, Resnik N, Veranič P, Franceschi P, Sepčić K, Guella G (2016) Comparative lipidomic study of urothelial cancer models: association with urothelial cancer cell invasiveness. Mol Biosyst 12(11):3266–3279. https://doi.org/10.1039/c6mb00477f
Yu T, An Q, Cao XL, Yang H, Cui J, Li ZJ, Xiao G (2020) GOLPH3 inhibition reverses oxaliplatin resistance of colon cancer cells via suppression of PI3K/AKT/mTOR pathway. Life Sci 260:118294. https://doi.org/10.1016/j.lfs.2020.118294
Zhang X (2021) Alterations of Golgi structural proteins and glycosylation defects in cancer. Front Cell Dev Biol 9:665289. https://doi.org/10.3389/fcell.2021.665289
Zhang Q, Zhuang J, Deng Y, Zhao X, Tang B, Yao D, Zhao W, Chang C, Lu Q, Chen W, Zhang S, Ji C, Cao L, Guo H (2015) GOLPH3 is a potential therapeutic target and a prognostic indicator of poor survival in bladder cancer treated by cystectomy. Oncotarget 6(31):32177–32192. https://doi.org/10.18632/oncotarget.4867
Zhang K, Sun Z, Chen X, Zhang Y, Guo A, Zhang Y (2021) Intercellular transport of tau protein and β-amyloid mediated by tunneling nanotubes. Am J Transl Res 13(11):12509–12522
Zupancic D, Romih R (2013) Heterogeneity of uroplakin localization in human normal urothelium, papilloma and papillary carcinoma. Radiol Oncol 47(4):338–345. https://doi.org/10.2478/raon-2013-0052
Zupančič D, Zakrajšek M, Zhou G, Romih R (2011) Expression and localization of four uroplakins in urothelial preneoplastic lesions. Histochem Cell Biol 136(4):491–500. https://doi.org/10.1007/s00418-011-0857-4
Zupančič D, Kreft ME, Romih R (2014) Selective binding of lectins to normal and neoplastic urothelium in rat and mouse bladder carcinogenesis models. Protoplasma 251(1):49–59. https://doi.org/10.1007/s00709-013-0524-9
Acknowledgements
We cordially thank Dr. G.V. Beznoussenko, Prof. M. Pavelka and Prof. R. Romih for the help in imaging and many Golgi discussions.
Funding
We acknowledge Slovenian Research Agency (ARRS) (Grant No. J7-2594 and P3-0108), INTAS (Project: 99-4-1732), Telethon (Project: E.1105), Consorzio Mario Negri, Italy, the Italian National Research Council (Convenzione CNR–CMN Sud) and the MRIC UL IP-0510 Infrastructure program for financial support.
Author information
Authors and Affiliations
Contributions
Conceptualization: MEK, AAM, and SH; Imaging: MEK, AAM, and SH; Writing–Original Draft Preparation: MEK; Writing–Review and Editing: MEK, AAM, and SH; All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kreft, M.E., Mironov, A.A. & Hudoklin, S. The Golgi complex: An organelle that determines urothelial cell biology in health and disease. Histochem Cell Biol 158, 229–240 (2022). https://doi.org/10.1007/s00418-022-02121-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-022-02121-0