Abstract
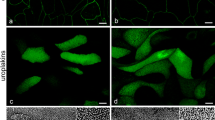
Optimizing culture conditions is known to be crucial for the differentiation of urothelial cell cultures and the formation of the permeability barrier. However, so far, no data exist to confirm if air–liquid (AL) and liquid–liquid (LL) interfaces are physiologically relevant during urothelial differentiation and barrier formation. To reveal the influence of interfaces on the proliferation, differentiation, and barrier formation of the urothelial cells (UCs) in vitro, we cultured UCs under four different conditions, i.e., at the AL or LL interfaces with physiological calcium concentration and without serum or without physiological calcium concentration and with serum. For each of the four models, the urothelial integrity was tested by measuring the transepithelial resistance (TER), and the differentiation stage was examined by immunolabeling of differentiation-related markers and ultrastructural analysis. We found that the UCs at a LL interface, regardless of the presence or absence of calcium or serum, form the urothelium with more cell layers and achieve a higher TER than UCs at an AL interface. However, UCs grown at an AL interface with physiological concentration of calcium in medium form only one- to two-layered urothelium of UCs, which are larger and express more differentiation-related proteins uroplakins than UCs in other models. These results demonstrate that the interface itself can play a major, although so-far neglected, role in urothelial physiology, particularly in the formation of the urothelial permeability barrier in vitro and the regulatory mechanisms related with urothelial differentiation. In the study, the culturing of UCs in three successive steps is proposed.




Similar content being viewed by others
References
Abraham G.; Zizzadoro C.; Kacza J.; Ellenberger C.; Abs V.; Franke J.; Schoon H. A.; Seeger J.; Tesfaigzi Y.; Ungemach F. R. Growth and differentiation of primary and passaged equine bronchial epithelial cells under conventional and air–liquid-interface culture conditions. BMC Vet Res 7: 26; 2011.
Acharya P.; Beckel J.; Ruiz W. G.; Wang E.; Rojas R.; Birder L.; Apodaca G. Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and −12 in bladder epithelium. Am J Physiol Renal Physiol 287: F305–318; 2004.
Bebok Z.; Tousson A.; Schwiebert L. M.; Venglarik C. J. Improved oxygenation promotes CFTR maturation and trafficking in MDCK monolayers. Am J Physiol Cell Physiol 280: C135–145; 2001.
Chang J. E.; Basu S. K.; Lee V. H. Air-interface condition promotes the formation of tight corneal epithelial cell layers for drug transport studies. Pharm Res 17: 670–676; 2000.
Cross W. R.; Eardley I.; Leese H. J.; Southgate J. A biomimetic tissue from cultured normal human urothelial cells: analysis of physiological function. Am J Physiol Renal Physiol 289: F459–468; 2005.
Gauvin R.; Larouche D.; Marcoux H.; Guignard R.; Auger FA.; Germain L. Minimal contraction for tissue-engineered skin substitutes when matured at the air–liquid interface. J Tissue Eng Regen Med. doi:10.1002/term.543; 2012.
Gu Y.; Wang C.; Cohen A. Effect of IGF-1 on the balance between autophagy of dysfunctional mitochondria and apoptosis. FEBS Lett 577: 357–360; 2004.
Guhe C.; Follmann W. Growth and characterization of porcine urinary bladder epithelial cells in vitro. Am J Physiol 266: F298–308; 1994.
Hicks R. M. The mammalian urinary bladder: an accommodating organ. Biol Rev Camb Philos Soc 50: 215–246; 1975.
Hu P.; Meyers S.; Liang F. X.; Deng F. M.; Kachar B.; Zeidel M. L.; Sun T. T. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283: F1200–1207; 2002.
Kreft M. E.; Di Giandomenico D.; Beznoussenko G. V.; Resnik N.; Mironov A. A.; Jezernik K. Golgi apparatus fragmentation as a mechanism responsible for uniform delivery of uroplakins to the apical plasma membrane of uroepithelial cells. Biol Cell 102: 593–607; 2010.
Kreft M. E.; Hudoklin S.; Sterle M. Establishment and characterization of primary and subsequent subcultures of normal mouse urothelial cells. Folia Biol (Praha) 51: 126–132; 2005b.
Kreft M. E.; Jezernik K.; Kreft M.; Romih R. Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann N Y Acad Sci 1152: 18–29; 2009a.
Kreft M. E.; Robenek H. Freeze-fracture replica immunolabelling reveals urothelial plaques in cultured urothelial cells. PloS One 7: e38509; 2012.
Kreft M. E.; Romih R.; Kreft M.; Jezernik K. Endocytotic activity of bladder superficial urothelial cells is inversely related to their differentiation stage. Differentiation 77: 48–59; 2009b.
Kreft M. E.; Romih R.; Sterle M. Antigenic and ultrastructural markers associated with urothelial cytodifferentiation in primary explant outgrowths of mouse bladder. Cell Biol Int 26: 63–74; 2002.
Kreft M. E.; Sterle M.; Jezernik K. Distribution of junction- and differentiation-related proteins in urothelial cells at the leading edge of primary explant outgrowths. Histochem Cell Biol 125: 475–485; 2006.
Kreft M. E.; Sterle M.; Veranic P.; Jezernik K. Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol 123: 529–539; 2005a.
Lee M. K.; Yoo J. W.; Lin H.; Kim Y. S.; Kim D. D.; Choi Y. M.; Park S. K.; Lee C. H.; Roh H. J. Air–liquid interface culture of serially passaged human nasal epithelial cell monolayer for in vitro drug transport studies. Drug Deliv 12: 305–311; 2005.
Lewis S. A.; Diamond J. M. Na+ transport by rabbit urinary bladder, a tight epithelium. J Membr Biol 28: 1–40; 1976.
Liang F. X.; Riedel I.; Deng F. M.; Zhou G.; Xu C.; Wu X. R.; Kong X. P.; Moll R.; Sun T. T. Organization of uroplakin subunits: transmembrane topology, pair formation and plaque composition. Biochem J 355: 13–18; 2001.
Lin H.; Li H.; Cho H. J.; Bian S.; Roh H. J.; Lee M. K.; Kim J. S.; Chung S. J.; Shim C. K.; Kim D. D. Air–liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J Pharm Sci 96: 341–350; 2007.
Miessen K.; Einspanier R.; Schoen J. Establishment and characterization of a differentiated epithelial cell culture model derived from the porcine cervix uteri. BMC Vet Res 8: 31; 2012.
Miessen K.; Sharbati S.; Einspanier R.; Schoen J. Modelling the porcine oviduct epithelium: a polarized in vitro system suitable for long-term cultivation. Theriogenology 76: 900–910; 2011.
Negrete H. O.; Lavelle J. P.; Berg J.; Lewis S. A.; Zeidel M. L. Permeability properties of the intact mammalian bladder epithelium. Am J Physiol 271: F886–894; 1996.
Nossol C.; Diesing A. K.; Walk N.; Faber-Zuschratter H.; Hartig R.; Post A.; Kluess J.; Rothkötter H. J.; Kahlert S. Air–liquid interface cultures enhance the oxygen supply and trigger the structural and functional differentiation of intestinal porcine epithelial cells (IPEC). Histochem Cell Biol 136: 103–115; 2011.
Pariente J. L.; Bordenave L.; Bareille R.; Baquey C.; Le Guillou M. Cultured differentiated human urothelial cells in the biomaterials field. Biomaterials 21: 835–839; 2000.
Ram-Liebig G.; Meye A.; Hakenberg O. W.; Haase M.; Baretton G.; Wirth M. P. Induction of proliferation and differentiation of cultured urothelial cells on acellular biomaterials. BJU Int 94: 922–927; 2004.
Rodier F.; Campisi J. Four faces of cellular senescence. J Cell Biol 192: 547–556; 2011.
Romih R.; Korosec P.; de Mello W.; Jr J. K. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res 320: 259–268; 2005.
Southgate J.; Hutton K. A.; Thomas D. F.; Trejdosiewicz L. K. Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest 71: 583–594; 1994.
Truschel S. T.; Ruiz W. G.; Shulman T.; Pilewski J.; Sun T. T.; Zeidel M. L.; Apodaca G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem 274: 15020–15029; 1999.
Turner A. M.; Subramaniam R.; Thomas D. F.; Southgate J. Generation of a functional, differentiated porcine urothelial tissue in vitro. Eur Urol 54: 1423–1432; 2008.
Varley C. L.; Southgate J. Organotypic and 3D reconstructed cultures of the human bladder and urinary tract. Methods Mol Biol 695: 197–211; 2011.
Veranic P.; Jezernik K. Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil Cytoskeleton 53: 317–325; 2002.
Veranic P.; Romih R.; Jezernik K. What determines differentiation of urothelial umbrella cells? Eur J Cell Biol 83: 27–34; 2004.
Visnjar T.; Kocbek P.; Kreft M. E. Hyperplasia as a mechanism for rapid resealing urothelial injuries and maintaining high transepithelial resistance. Histochem Cell Biol 137: 177–186; 2012.
Wu X. R.; Manabe M.; Yu J.; Sun T. T. Large scale purification and immunolocalization of bovine uroplakins I, II, and III. Molecular markers of urothelial differentiation. J Biol Chem 265: 19170–19179; 1990.
Acknowledgments
We thank Prof. Dr. Kristijan Jezernik and Prof. Dr. Rok Romih for their continuous support and Eva Lasič for checking the English of the manuscript. The study was supported by the Slovenian Research Agency ARRS (number P3-0108).
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
About this article
Cite this article
Višnjar, T., Kreft, M.E. Air–liquid and liquid–liquid interfaces influence the formation of the urothelial permeability barrier in vitro. In Vitro Cell.Dev.Biol.-Animal 49, 196–204 (2013). https://doi.org/10.1007/s11626-013-9585-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-013-9585-5