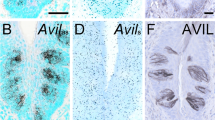
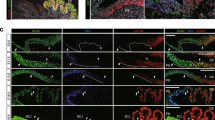
Abstract
Epithelial tuft cells are named after their characteristic microtubule bundles located at the cell apex where these are exposed to the luminal environment. As such, tuft cells are found in multiple organs, including the gastrointestinal (GI) tract where the apical “tuft” is hypothesized to detect and transmit environmental signals. Thus, the goal of our study was to characterize gastric tuft cells during GI tract development, then subsequently in the normal and metaplastic adult stomach. GI tracts from mouse embryos, and newborn and postnatal mice were analyzed. Tuft cells were identified by immunohistochemistry using acetylated-α-tubulin (acTub) antibody to detect the microtubule bundle. Additional tuft cell markers, e.g., doublecortin-like kinase 1 (DCLK1), were used to co-localize with acTub. Tuft cells were quantified in human gastric tissue arrays and in mouse stomachs with or without inflammation. In the developing intestine, tuft cells in both the crypts and villi expressed all markers by E18.5. In the stomach, acTub co-localized with DCLK1 and other established tuft cell markers by E18.5 in the antrum, but not until postnatal day 7 in the corpus, with the highest density of tuft cells clustered at the forestomach ridge. Tuft cell numbers increased in hyperplastic human and mouse stomachs. In the adult GI tract, the tuft cell marker acTub co-expressed with DCKL1 and chemosensory markers, e.g.,TRPM5. In summary, tuft cells appear in the gastric antrum and intestine at E18.5, but their maximal numbers in the corpus are not achieved until after weaning. Tuft cell numbers increase with inflammation, hyperplasia, and metaplasia.






Similar content being viewed by others
References
Barker N, Huch M, Kujala P, van de Wetering M et al (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6:25–36
Elia G, Chinery R, Hanby AM, Poulsom R et al (1994) The production and characterization of a new monoclonal antibody to the trefoil peptide human spasmolytic polypeptide. Histochem J 26:644–647
Friis-Hansen L, Sundler F, Li Y, Gillespie PJ et al (1998) Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol 274:G561–G568
Gerbe F, Brulin B, Makrini L, Legraverend C et al (2009) DCAMKL-1 expression identifies tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 137:2179–2180 (author reply 2180-2171)
Gerbe F, van Es JH, Makrini L, Brulin B et al (2011) Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192:767–780
Giannakis M, Stappenbeck TS, Mills JC, Leip DG et al (2006) Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem 281:11292–11300
Hansen A, Finger TE (2008) Is TrpM5 a reliable marker for chemosensory cells? Multiple types of microvillous cells in the main olfactory epithelium of mice. BMC Neurosci 9:115
Hass N, Schwarzenbacher K, Breer H (2007) A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol 128:457–471
Hass N, Schwarzenbacher K, Breer H (2010) T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 339:493–504
Hofer D, Drenckhahn D (1996) Cytoskeletal markers allowing discrimination between brush cells and other epithelial cells of the gut including enteroendocrine cells. Histochem Cell Biol 105:405–412
Hofer D, Drenckhahn D (1998) Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol 110:303–309
Howard TA, Misra DN, Grove M, Becich MJ et al (1996) Human gastric intrinsic factor expression is not restricted to parietal cells. J Anat 189(Pt 2):303–313
Iseki S (1991) Postnatal development of the brush cells in the common bile duct of the rat. Cell Tissue Res 266:507–510
Iseki S, Kondo H (1989) Specific localization of hepatic fatty acid-binding protein in the gastric brush cells of rats. Cell Tissue Res 257:545–548
Iseki S, Kanda T, Hitomi M, Ono T (1991) Ontogenic appearance of three fatty acid binding proteins in the rat stomach. Anat Rec 229:51–60
Isomaki AM (1973) A new cell type (tuft cell) in the gastrointestinal mucosa of the rat. A transmission and scanning electron microscopic study. Acta Pathol Microbiol Scand A 240(Suppl):235–241
Jain RN, Al-Menhali AA, Keeley TM, Ren J et al (2008) Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest 118:2459–2470
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD et al (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074
Jarvi O, Keyrilainen O (1956) On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand 39(Suppl):72–73
Jin G, Ramanathan V, Quante M, Baik GH et al (2009) Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest 119:2691–2701
Judd LM, Andringa A, Rubio CA, Spicer Z et al (2005) Gastric achlorhydria in H/K-ATPase-deficient (Atp4a(−/−)) mice causes severe hyperplasia, mucocystic metaplasia and upregulation of growth factors. J Gastroenterol Hepatol 20:1266–1278
Kang W, Saqui-Salces M, Zavros Y, Merchant JL (2008) Induction of follistatin precedes gastric transformation in gastrin deficient mice. Biochem Biophys Res Commun 376:573–577
Karam SM, Leblond CP (1993) Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec 236:333–340
Kaske S, Krasteva G, Konig P, Kummer W et al (2007) TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8:49
Kasper M, Hofer D, Woodcock-Mitchell J, Migheli A et al (1994) Colocalization of cytokeratin 18 and villin in type III alveolar cells (brush cells) of the rat lung. Histochemistry 101:57–62
Keeley TM, Samuelson LC (2010) Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol 299:G1241–G1251
Kikuchi M, Nagata H, Watanabe N, Watanabe H et al (2010) Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia and dysplasia. BMC Gastroenterol 10:65
Kokrashvili Z, Rodriguez D, Yevshayeva V, Zhou H et al (2009) Release of endogenous opioids from duodenal enteroendocrine cells requires Trpm5. Gastroenterology 137:598–606 606 e591-592
Lin W, Ezekwe EA Jr, Zhao Z, Liman ER et al (2008) TRPM5-expressing microvillous cells in the main olfactory epithelium. BMC Neurosci 9:114
Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y et al (2006) Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol 290:G970–G979
Luciano L, Reale E (1979) A new morphological aspect of the brush cells of the mouse gallbladder epithelium. Cell Tissue Res 201:37–44
Luciano L, Reale E (1990) Brush cells of the mouse gallbladder. A correlative light- and electron-microscopical study. Cell Tissue Res 262:339–349
Luciano L, Castellucci M, Reale E (1981) The brush cells of the common bile duct of the rat. This section, freeze-fracture and scanning electron microscopy. Cell Tissue Res 218:403–420
May R, Riehl TE, Hunt C, Sureban SM et al (2008) Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26:630–637
May R, Sureban SM, Hoang N, Riehl TE et al (2009) Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 27:2571–2579
Mensah-Osman E, Zavros Y, Merchant JL (2008) Somatostatin stimulates menin gene expression by inhibiting protein kinase A. Am J Physiol Gastrointest Liver Physiol 295:G843–G854
Nabeyama A, Leblond CP (1974) “Caveolated cells” characterized by deep surface invaginations and abundant filaments in mouse gastro-intestinal epithelia. Am J Anat 140:147–165
Nakamura E, Hasumura M, San Gabriel A, Uneyama H et al (2010) New frontiers in gut nutrient sensor research: luminal glutamate-sensing cells in rat gastric mucosa. J Pharmacol Sci 112:13–18
Norwood MG, Bailey N, Nanji M, Gillies RS et al (2010) Cytoplasmic beta-catenin accumulation is a good prognostic marker in upper and lower gastrointestinal adenocarcinomas. Histopathology 57:101–111
Ogura T, Krosnowski K, Zhang L, Bekkerman M et al (2010) Chemoreception regulates chemical access to mouse vomeronasal organ: role of solitary chemosensory cells. PLoS One 5:e11924
Okumura T, Ericksen RE, Takaishi S, Wang SS et al (2010) K-ras mutation targeted to gastric tissue progenitor cells results in chronic inflammation, an altered microenvironment, and progression to intraepithelial neoplasia. Cancer Res 70:8435–8445
Rau T, Dimmler A, Hafner M, Brabletz T et al (2005) Aberrant expression of TTF-1 and forkhead factor HFH-4 in atrophic gastritis and ciliated metaplasia suggests gastric broncho-pulmonary transdetermination. J Pathol 206:383–387
Rozengurt N, Wu SV, Chen MC, Huang C et al (2006) Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291:G792–G802
Saha S, Slepecky NB (2000) Age-related changes in microtubules in the guinea pig organ of Corti. Tubulin isoform shifts with increasing age suggest changes in micromechanical properties of the sensory epithelium. Cell Tissue Res 300:29–46
Sato A, Miyoshi S (1997) Fine structure of tuft cells of the main excretory duct epithelium in the rat submandibular gland. Anat Rec 248:325–331
Sato A, Hamano M, Miyoshi S (1998) Increasing frequency of occurrence of tuft cells in the main excretory duct during postnatal development of the rat submandibular gland. Anat Rec 252:276–280
Sato A, Hisanaga Y, Inoue Y, Nagato T et al (2002) Three-dimensional structure of apical vesicles of tuft cells in the main excretory duct of the rat submandibular gland. Eur J Morphol 40:235–239
Sbarbati A, Osculati F (2005) A new fate for old cells: brush cells and related elements. J Anat 206:349–358
Sbarbati A, Crescimanno C, Benati D, Osculati F (1998) Solitary chemosensory cells in the developing chemoreceptorial epithelium of the vallate papilla. J Neurocytol 27:631–635
Sbarbati A, Merigo F, Osculati F (2010) Eukaryotic vs. prokaryotic chemosensory systems. Biomed Pharmacother 64:233–239
Shah AS, Ben-Shahar Y, Moninger TO, Kline JN et al (2009) Motile cilia of human airway epithelia are chemosensory. Science 325:1131–1134
Slepecky NB, Henderson CG, Saha S (1995) Post-translational modifications of tubulin suggest that dynamic microtubules are present in sensory cells and stable microtubules are present in supporting cells of the mammalian cochlea. Hear Res 91:136–147
Waghray M, Zavros Y, Saqui-Salces M, El-Zaatari M et al (2010) Interleukin-1beta promotes gastric atrophy through suppression of Sonic Hedgehog. Gastroenterology 138:562–572 (572 e561–e562)
Xiao C, Ogle SA, Schumacher MA, Orr-Asman MA et al (2010) Loss of parietal cell expression of sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138:550–561
Yee CL, Yang R, Bottger B, Finger TE et al (2001) “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol 440:97–108
Young RL, Sutherland K, Pezos N, Brierley SM et al (2009) Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut 58:337–346
Zavros Y, Eaton KA, Kang W, Rathinavelu S et al (2005) Chronic gastritis in the hypochlorhydric gastrin-deficient mouse progresses to adenocarcinoma. Oncogene 24:2354–2366
Zhang Y, Huang X (2010) Investigation of doublecortin and calcium/calmodulin-dependent protein kinase-like-1-expressing cells in the mouse stomach. J Gastroenterol Hepatol 25:576–582
Acknowledgments
This study was supported by NIH Grant P01-DK62041 (J.L.M.), NIH Grants RO1-DK078926 (L.C.S.), and University of Michigan Digestive Disease Center Grant P30-DK-34933.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 2
: Co-localization of acTub (red) with known tuft cells markers: (A) guanine nucleotide binding protein α-transducing 3 (GNAT3, also called α-gustducin, green) and (B) Villin-1 (green) shown in an isolated gland prep from the squamous/columnar epithelium border region. The basal membranes are indicated with white dashed lines in panel A. C) Glucagon-like peptide 1 (GLP-1) (green) also co-localized with acTub (red) in tuft cells. (TIFF 4462 kb)
Rights and permissions
About this article
Cite this article
Saqui-Salces, M., Keeley, T.M., Grosse, A.S. et al. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol 136, 191–204 (2011). https://doi.org/10.1007/s00418-011-0831-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-011-0831-1