Abstract
Purpose
MicroRNA-93 (miR-93) usually acts as a promoter of tumor progression in several human carcinomas. It has been found distinctly high in eyes with proliferative diabetic retinopathy (DR). The present study aims to investigate the role of plasma miR-93 in the progression of type 2 diabetic retinopathy (T2DR).
Methods
Our study subjects were made up of 140 type 2 diabetes mellitus (T2DM) patients who were assigned into DR (DR patients, n = 75), NDR (non-DR patients, n = 65), and control (healthy individuals, n = 127) groups. Levels of fasting blood glucose (FBG), fasting plasma glucose (FPG), triglyceride (TG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), blood urea nitrogen (BUN), creatinine (Cr), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting insulin (FIsn) were detected by automatic biochemical analyzer. Enzyme-linked immunosorbent assay (ELISA) was performed for the levels of interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and vascular endothelial growth factor (VEGF), qRT-PCR for the miR-93 expression in plasma, and mRNA expressions of IL-1, IL-6, TNF-α and VEGF; receiver operating characteristic (ROC) curve for the diagnostic performance of miR-93 to T2DR, Pearson correlation analysis for correlation analysis between miR-93 and other indexes detected before and multivariate logistic regression analyses for the risk factors for T2DR.
Results
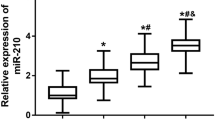
The DR and NDR groups exhibited elevated course of disease, and decreased levels of FBG, FPG, TG, HbA1c, TC, BUN, Cr, HDL-C, FIsn, IL-1, IL-6, TNF-α and VEGF but declined LDL-C level as compared to the control group. The course of disease was longer and the levels of FBG, FPG, HbA1c, IL1, IL6 and VEGF were higher in the DR group than those in the NDR group (all P < 0.05). The miR-93 expression and RNA expressions of IL-1, IL-6, TNF-α and VEGF were higher in the DR group than those in the NDR group (P < 0.05). The best cutoff for miR-93 to assess T2DR was 1.31, with a Youden index of 0.63, sensitivity of 73.33%, specificity of 89.24%, and area under the curve (AUC) of 0.866. Pearson correlation analysis indicated that miR-93 expression was positively associated with course of disease, the levels of FPG, HbA1c, TNF-α and VEGF. T2DM patients with longer disease course, higher levels of FBG, HbA1c, VEGF and miR-93 expression were risk factors for developing DR.
Conclusion
Our study demonstrates that plasma miR-93 is associated with the progression of T2DR and it can sever as a diagnostic marker for T2DR.



Similar content being viewed by others
References
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136. doi:10.1016/S0140-6736(09)62124-3
Burdon KP, Fogarty RD, Shen W, Abhary S, Kaidonis G, Appukuttan B, Hewitt AW, Sharma S, Daniell M, Essex RW, Chang JH, Klebe S, Lake SR, Pal B, Jenkins A, Govindarjan G, Sundaresan P, Lamoureux EL, Ramasamy K, Pefkianaki M, Hykin PG, Petrovsky N, Brown MA, Gillies MC, Craig JE (2015) Genome-wide association study for sight-threatening diabetic retinopathy reveals association with genetic variation near the GRB2 gene. Diabetologia 58:2288–2297. doi:10.1007/s00125-015-3697-2
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease Study G (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. doi:10.2337/dc11-1909
Ding J, Wong TY (2012) Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 12:346–354. doi:10.1007/s11892-012-0283-6
Yang JK, Liu W, Shi J, Li YB (2010) An association between subclinical hypothyroidism and sight-threatening diabetic retinopathy in type 2 diabetic patients. Diabetes Care 33:1018–1020. doi:10.2337/dc09-1784
Das A, Stroud S, Mehta A, Rangasamy S (2015) New treatments for diabetic retinopathy. Diabetes Obes Metab 17:219–230. doi:10.1111/dom.12384
Kollias AN, Ulbig MW (2010) Diabetic retinopathy: early diagnosis and effective treatment. Dtsch Arztebl Int 107:75–83. doi:10.3238/arztebl.2010.0075, quiz 84
Pandey AK, Agarwal P, Kaur K, Datta M (2009) MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem 23:221–232. doi:10.1159/000218169
Herrera BM, Lockstone HE, Taylor JM, Ria M, Barrett A, Collins S, Kaisaki P, Argoud K, Fernandez C, Travers ME, Grew JP, Randall JC, Gloyn AL, Gauguier D, McCarthy MI, Lindgren CM (2010) Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53:1099–1109. doi:10.1007/s00125-010-1667-2
Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L (2014) Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res 43:92–107. doi:10.1016/j.preteyeres.2014.07.003
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, O’Malley YQ, Askeland RW, Sugg S, Liu M, Mehta T, Deng Z, Yang BB (2012) MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle 11:4352–4365. doi:10.4161/cc.22670
Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, Yang BB (2011) MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene 30:806–821. doi:10.1038/onc.2010.465
Tang Q, Zou Z, Zou C, Zhang Q, Huang R, Guan X, Li Q, Han Z, Wang D, Wei H, Gao X, Wang X (2015) MicroRNA-93 suppress colorectal cancer development via Wnt/beta-catenin pathway downregulating. Tumour Biol 36:1701–1710. doi:10.1007/s13277-014-2771-6
Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, Chen BS, Chazenbalk G, Azziz R (2013) miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62:2278–2286. doi:10.2337/db12-0963
Hirota K, Keino H, Inoue M, Ishida H, Hirakata A (2015) Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 253:335–342. doi:10.1007/s00417-014-2692-5
Daniiarov SB, Zarif’ian AG (1974) Effect of nuredal on frequency of cardiac contractions, catecholamines and tolerance of rats to pressure chamber anoxia under conditions of varied altitude. Sov Zdravookhr Kirg 1:3–10
Ding J, Strachan MW, Reynolds RM, Frier BM, Deary IJ, Fowkes FG, Lee AJ, McKnight J, Halpin P, Swa K, Price JF, Edinburgh Type 2 Diabetes Study I (2010) Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 59:2883–2889. doi:10.2337/db10-0752
Raczynska D, Zorena K, Urban B, Zalewski D, Skorek A, Malukiewicz G, Sikorski BL (2014) Current trends in the monitoring and treatment of diabetic retinopathy in young adults. Mediat Inflamm 2014:492926. doi:10.1155/2014/492926
Kantharidis P, Wang B, Carew RM, Lan HY (2011) Diabetes complications: the microRNA perspective. Diabetes 60:1832–1837. doi:10.2337/db11-0082
Group AS, Group AES, Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine L (2010) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363:233–244. doi:10.1056/NEJMoa1001288
Emerging Risk Factors C, Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841. doi:10.1056/NEJMoa1008862
Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, Albright AL, Cowie CC, Klein R, Saaddine JB (2009) Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: implications for diabetes diagnostic thresholds. Diabetes Care 32:2027–2032. doi:10.2337/dc09-0440
Izuta H, Chikaraishi Y, Adachi T, Shimazawa M, Sugiyama T, Ikeda T, Hara H (2009) Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients. Mol Vis 15:2663–2672
Selim KM, Sahan D, Muhittin T, Osman C, Mustafa O (2010) Increased levels of vascular endothelial growth factor in the aqueous humor of patients with diabetic retinopathy. Indian J Ophthalmol 58:375–379. doi:10.4103/0301-4738.67042
Gao X, Li Y, Wang H, Li C, Ding J (2016) Inhibition of HIF-1alpha decreases expression of pro-inflammatory IL-6 and TNF-alpha in diabetic retinopathy. Acta Ophthalmol. doi:10.1111/aos.13096doi
Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X, Yang D (2015) MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab 100:E729–E738. doi:10.1210/jc.2014-3827
Salam A, Mathew R, Sivaprasad S (2011) Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmol 89:405–411. doi:10.1111/j.1755-3768.2010.02079.x
Long J, Wang Y, Wang W, Chang BH, Danesh FR (2010) Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem 285:23457–23465. doi:10.1074/jbc.M110.136168
Gong JY, Sun YH (2013) Association of VEGF gene polymorphisms with diabetic retinopathy: a meta-analysis. PLoS One 8, e84069. doi:10.1371/journal.pone.0084069
He J, Wang H, Liu Y, Li W, Kim D, Huang H (2015) Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. J Ophthalmol 2015:605946. doi:10.1155/2015/605946
Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N (2011) Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med 171:404–410. doi:10.1001/archinternmed.2011.2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Suqian provided financial support in the form of a grant through the Suqian Science and Technology Support Program (S201415). The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Yan Wang is regarded as co-first author.
Rights and permissions
About this article
Cite this article
Zou, HL., Wang, Y., Gang, Q. et al. Plasma level of miR-93 is associated with higher risk to develop type 2 diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255, 1159–1166 (2017). https://doi.org/10.1007/s00417-017-3638-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3638-5