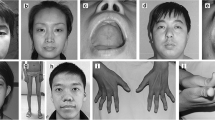
Abstract
Centronuclear myopathies (CNMs) are a group of clinically and genetically heterogeneous muscle disorders. To date, mutation in 7 different genes has been reported to cause CNMs but 30 % of cases still remain genetically undefined. Genetic investigations are often expensive and time consuming. Clinical and morphological clues are needed to facilitate genetic tests and to choose the best approach for genetic screening. We aimed to describe genotype–phenotype correlation in an Italian cohort of patients affected by CNMs, to define the relative frequencies of its defined genetic forms and to draw a diagnostic algorithm to address genetic investigations. We recruited patients with CNMs from all the Italian tertiary neuromuscular centers following clinical and histological criteria. All selected patients were screened for the four ‘canonical’ genes related to CNMs: MTM1, DNM2, RYR1 and BIN1. Pathogenetic mutations were found in 38 of the 54 screened patients (70 %), mostly in patients with congenital onset (25 of 30 patients, 83 %): 15 in MTM1, 6 in DNM2, 3 in RYR1 and one in TTN. Among the 13 patients with a childhood–adolescence onset, mutations were found in 6 patients (46 %), all in DNM2. In the group of the 11 patients with adult onset, mutations were identified in 7 patients (63 %), again in DNM2, confirming that variants in this gene are relatively more common in late-onset phenotypes. The present study provides the relative molecular frequency of centronuclear myopathy and of its genetically defined forms in Italy and also proposes a diagnostic algorithm to be used in clinical practice to address genetic investigations.





Similar content being viewed by others
References
Romero NB (2010) Centronuclear myopathies: a widening concept. Neuromuscul Disord 20:223–228
Romero NB, Bitoun M (2011) Centronuclear myopathies. Semin Pediatr Neurol 18:250–256
Böhm J, Biancalana V, Malfatti E et al (2014) Adult-onset autosomal dominant centronuclear myopathy due to BIN1 mutations. Brain 137(12):3160–3170
Bharucha-Goebel DX, Santi M, Medne L et al (2013) Severe congenital RYR1-associated myopathy: the expanding clinicopathologic and genetic spectrum. Neurology 80(17):1584–1589
Nicot AS, Toussaint A, Tosch V et al (2007) Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 39(9):1134–1139
Toussaint A, Cowling BS, Hnia K et al (2011) Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol 121(2):253–266
Wilmshurst JM, Lillis S, Zhou H et al (2010) RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol 68(5):717–726
Carmignac V, Salih MA, Quijano-Roy S et al (2007) C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol 61(4):340–351
Ceyhan-Birsoy O, Agrawal PB, Hidalgo C et al (2013) Recessive truncating titin gene, TTN, mutations presenting as centronuclear myopathy. Neurology 81(14):1205–1214
Agrawal PB, Pierson CR, Joshi M et al (2014) SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am J Hum Genet 95(2):218–226
Majczenko K, Davidson AE, Camelo-Piragua S et al (2012) Dominant mutation of CCDC78 in a unique congenital myopathy with prominent internal nuclei and atypical cores. Am J Hum Genet 91(2):365–371
North KN, Wang CH, Clarke N et al (2014) International Standard of Care Committee for Congenital Myopathies. Approach to the diagnosis of congenital myopathies. Neuromuscul Disord 24(2):97–116
Dubowitz V, Sewry CA (2007) Muscle biopsy: a practical approach. 3rd ed. Sanders/Elsevier, Edinburgh
Hackman P, Marchand S, Sarparanta J et al (2008) Truncating mutations in C-terminal titin may cause more severe tibial muscular dystrophy (TMD). Neuromuscul Disord 18(12):922–928
Evilä A, Vihola A, Sarparanta J et al (2014) Atypical phenotypes in titinopathies explained by second titin mutations. Ann Neurol 75(2):230–240
Tasca G, Fattori F, Ricci E et al (2013) Somatic mosaicism in TPM2-related myopathy with nemaline rods and cap structures. Acta Neuropathol 125(1):169–171
Catteruccia M, Fattori F, Codemo V et al (2013) Centronuclear myopathy related to dynamin 2 mutations: clinical, morphological, muscle imaging and genetic features of an Italian cohort. Neuromuscul Disord 23(3):229–238
Hwang JH, Zorzato F, Clarke NF, Treves S (2012) Mapping domains and mutations on the skeletal muscle ryanodine receptor channel. Trends Mol Med 18:644–657
Chauveau C, Rowell J, Ferreiro A (2014) A rising titan: TTN review and mutation update. Hum Mutat 35(9):1046–1059
Biancalana V, Beggs AH, Das S, et al (2012) Clinical utility gene card for: Centronuclear and myotubular myopathies. Eur J Hum Genet 20(10)
Smets K (2008) X-linked myotubular myopathy and chylothorax. Neuromuscul Disord 18:183–184
Oliveira J, Oliveira ME, Kress W et al (2013) Expanding the MTM1 mutational spectrum: novel variants including the first multi-exonic duplication and development of a locus-specific database. Eur J Hum Genet 21(5):540–549
McEntagart M, Parsons G, Buj-Bello A et al (2002) Genotype–phenotype correlations in X-linked myotubular myopathy. Neuromuscul Disord 12(10):939–946
Böhm J, Biancalana V, Dechene ET et al (2012) Mutation spectrum in the large GTPase dynamin 2, and genotype–phenotype correlation in autosomal dominant centronuclear myopathy. Hum Mutat 33(6):949–959
Jungbluth H, Sewry CA, Muntoni F (2011) Core myopathies. Semin Pediatr Neurol 18:239–249
Herman DS, Lam L, Taylor MRG et al (2012) Truncations of titin causing dilated cardiomyopathy. N Engl J Med 366:619–628
Bitoun M, Bevilacqua JA, Prudhon B et al (2007) Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol 62(6):666–670
Bevilacqua JA, Bitoun M, Biancalana V et al (2009) “Necklace” fibers, a new histological marker of late-onset MTM1-related centronuclear myopathy. Acta Neuropathol 117(3):283–291
Gurgel-Giannetti J, Zanoteli E, de Castro Concentino EL et al (2012) Necklace fibers as histopathological marker in a patient with severe form of X-linked myotubular myopathy. Neuromuscul Disord 22(6):541–545
Liewluck T, Lovell TL, Bite AV, Engel AG (2010) Sporadic centronuclear myopathy with muscle pseudohypertrophy, neutropenia, and necklace fibers due to a DNM2 mutation. Neuromuscul Disord 20(12):801–804
Bitoun M, Maugenre S, Jeannet PY et al (2005) Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet 37(11):1207–1209
Susman RD, Quijano-Roy S, Yang N et al (2010) Expanding the clinical, pathological and MRI phenotype of DNM2-related centronuclear myopathy. Neuromuscul Disord 20(4):229–237
Tanner SM, Schneider V, Thomas NS, Clarke A, Lazarou L, Liechti-Gallati S (1999) Characterization of 34 novel and six known MTM1 gene mutations in 47 unrelated X-linked myotubular myopathy patients. Neuromuscul Disord 9(1):41–49
Laporte J, Guiraud-Chaumeil C, Vincent MC et al (1997) Mutations in the MTM1 gene implicated in X-linked myotubular myopathy. ENMC International Consortium on Myotubular Myopathy. European Neuro-Muscular Center. Hum Mol Genet 6(9):1505–1511
Flex E, De Luca A, D’Apice MR, Buccino A, Dallapiccola B, Novelli G (2002) Rapid scanning of myotubularin (MTM1) gene by denaturing high-performance liquid chromatography (DHPLC). Neuromuscul Disord 12(5):501–505
De Luca A, Torrente I, Mangino M, Bertini E, Dallapiccola B, Novelli G (1999) A novel mutation (R271X) in the myotubularin gene causes a severe myotubular myopathy. Hum Hered 49(1):59–60
de Gouyon BM, Zhao W, Laporte J, Mandel JL, Metzenberg A, Herman GE (1997) Characterization of mutations in the myotubularin gene in twenty six patients with X-linked myotubular myopathy. Hum Mol Genet 6(9):1499–1504
Biancalana V, Caron O, Gallati S et al (2003) Characterisation of mutations in 77 patients with X-linked myotubular myopathy, including a family with a very mild phenotype. Hum Genet 112(2):135–142
Monnier N, Krivosic-Horber R, Payen JF et al (2002) Presence of two different genetic traits in malignant hyperthermia families: implication for genetic analysis, diagnosis, and incidence of malignant hyperthermia susceptibility. Anesthesiology 97(5):10–1067
Acknowledgments
The financial support of Telethon—Italy (Grant no. 08005) and a Grant of the Ministry of Health Finalizzata (code GR-2010-2310981) are gratefully acknowledged.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
All patients, parents, or healthy individuals provided written informed consent for genetic analyses and use of their anonymized data at the individual centers. This study has been performed in accordance with the ethical standards laid down in the 1965 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fattori, F., Maggi, L., Bruno, C. et al. Centronuclear myopathies: genotype–phenotype correlation and frequency of defined genetic forms in an Italian cohort. J Neurol 262, 1728–1740 (2015). https://doi.org/10.1007/s00415-015-7757-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7757-9