Abstract
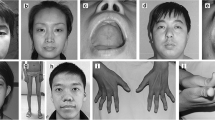
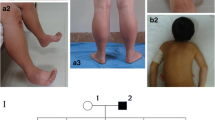
Mutations in the gene encoding the phosphoinositide phosphatase myotubularin 1 protein (MTM1) are usually associated with severe neonatal X-linked myotubular myopathy (XLMTM). However, mutations in MTM1 have also been recognized as the underlying cause of “atypical” forms of XLMTM in newborn boys, female infants, female manifesting carriers and adult men. We reviewed systematically the biopsies of a cohort of patients with an unclassified form of centronuclear myopathy (CNM) and identified four patients presenting a peculiar histological alteration in some muscle fibers that resembled a necklace (“necklace fibers”). We analyzed further the clinical and morphological features and performed a screening of the genes involved in CNM. Muscle biopsies in all four patients demonstrated 4–20% of fibers with internalized nuclei aligned in a basophilic ring (necklace) at 3 μm beneath the sarcolemma. Ultrastructurally, such necklaces consisted of myofibrils of smaller diameter, in oblique orientation, surrounded by mitochondria, sarcoplasmic reticulum and glycogen granules. In the four patients (three women and one man), myopathy developed in early childhood but was slowly progressive. All had mutations in the MTM1 gene. Two mutations have previously been reported (p.E404K and p.R241Q), while two are novel; a c.205_206delinsAACT frameshift change in exon 4 and a c.1234A>G mutation in exon 11 leading to an abnormal splicing and the deletion of nine amino acids in the catalytic domain of MTM1. Necklace fibers were seen neither in DNM2- or BIN1-related CNM nor in males with classical XLMTM. The presence of necklace fibers is useful as a marker to direct genetic analysis to MTM1 in CNM.




Similar content being viewed by others
References
Biancalana V, Caron O, Gallati S et al (2003) Characterisation of mutations in 77 patients with X-linked myotubular myopathy, including a family with a very mild phenotype. Hum Genet 112:135–142
Bitoun M, Bevilacqua JA, Eymard B, Fardeau M, Guicheney P, Romero NB (2009) An atypical phenotype of centronuclear myopathy due to a novel dynamin 2 mutation. Neurology 72(1)
Bitoun M, Bevilacqua JA, Prudhon B et al (2007) Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol 62:666–670
Bitoun M, Maugenre S, Jeannet P-Y et al (2005) Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet 37:1207–1209
Buj-Bello A, Fougerousse F, Schwab Y et al (2008) AAV-mediated intramuscular delivery of myotubularin corrects the myotubular myopathy phenotype in targeted murine muscle and suggests a function in plasma membrane homeostasis. Hum Mol Genet 17:2132–2143
Buj-Bello A, Laugel V, Messaddeq N et al (2002) The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci USA 9923:15060–15065
de Goede CGEL, Kinsley A, Kingston H, Tomlin PI, Hughes MI (2005) Muscle biopsy without centrally located nuclei in a male child with mild X-linked myotubular myopathy. Dev Med Child Neurol 47:835–837
de Gouyon BM, Zhao W, Laporte J, Mandel JL, Metzenberg A, Herman GE (1997) Characterization of mutations in the myotubularin gene in twenty six patients with X-linked myotubular myopathy. Hum Mol Genet 6:1499–1504
Fardeau M, Tomé F (1994) Congenital myopathies. In: Engel AG, Franzini-Armstrong C (eds) Myology, 2nd edn. McGraw Hill, New York, pp 1500–1505
Fischer D, Herasse M, Bitoun M et al (2006) Characterization of the muscle involvement in dynamin 2 related centronuclear myopathy. Brain 129:1463–1469
Gardner-Medwin D, Walton JN (1974) The clinical examination of the voluntary muscles. In: Walton JN (ed) Disorders of voluntary muscles. Churchill-Livingstone, Edinburgh, pp 517–560
Goryunov D, Nightingale A, Bornfleth L, Leung C, Liem RKH (2008) Multiple disease-linked myotubularin mutations cause NFL assembly defects in cultured cells and disrupt myotubularin dimerization. J Neurochem 104:1536–1552
Grogan PM, Tanner SM, Ørstavik KH et al (2005) Myopathy with skeletal asymmetry and hemidiaphragm elevation is caused by myotubularin mutations. Neurology 64:1638–1640
Hammans SR, Robinson DO, Moutou C et al (2000) A clinical and genetic study of a manifesting heterozygote with X-linked myotubular myopathy. Neuromuscul Disord 10:133–137
Herman GE, Kopacz K, Zhao W, Mills PL, Metzenberg A, Das S (2002) Characterization of mutations in fifty North American patients with X-linked myotubular myopathy. Hum Mutat 192:114–121
Hoffjan S, Thiels C, Vorgerd M, Neuen-Jacob E, Epplen J, Kress W (2006) Extreme phenotypic variability in a German family with X-linked myotubular myopathy associated with E404 K mutation in MTM1. Neuromuscul Disord 16:749–753
Jungbluth H, Sewry CA, Buj-Bello A et al (2003) Early and severe presentation of X-linked myotubular myopathy in a girl with skewed X-inactivation. Neuromuscul Disord 13:55–59
Jungbluth H, Zhou H, Sewry CA et al (2007) Centronuclear myopathy due to a de novo dominant mutation in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord 17:338–345
Laporte J, Biancalana V, Tanner S, Kress W, Schneider V, Wallgren-Pettersson C (2000) MTM1 mutations in X-linked myotubular myopathy. Hum Mutat 15:393–409
Laporte J, Guiraud-Chaumeil C, Vincent MC et al (1997) Mutations in the MTM1 gene implicated in X-linked myotubular myopathy. Hum Mol Genet 6:1505–1511
Laporte J, Hu LJ, Kretz C et al (1996) A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13:175–182
Li D, Gonzalez O, Bachinski LL, Roberts R (2000) Human protein tyrosine phosphatase-like gene: expression profile, genomic structure, and mutation analysis in families with ARVD. Gene 256:237–243
Marty I, Thevenon D, Scotto C et al (2000) Cloning and characterization of a new isoform of skeletal muscle triadin. J Biol Chem 275:8206–8212
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F (2007) Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging 25:433–440
Nicot AS, Toussaint A, Tosch V et al (2007) Mutations in amphiphysin 2 BIN1 disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 33:1134–1139
Oldfors A, Kyllerman M, Wahlström J, Darnfors C, Henriksson KG (1989) X-linked myotubular myopathy: clinical and pathological findings in a family. Clin Genet 361:5–14
Pénisson-Besnier I, Biancalana V, Reynier P, Cossée M, Dubas F (2007) Diagnosis of myotubular myopathy in the oldest known manifesting female carrier: a clinical and genetic study. Neuromuscul Disord 172:180–185
Pierson CR, Tomczak K, Agrawal P, Moghadaszadeh B, Beggs AH (2005) X-linked myotubular and centronuclear myopathies. J Neuropathol Exp Neurol 64(7):555–564
Ralston E, Lu Z, Biscocho N et al (2006) Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J Cell Physiol 209:874–882
Schara U, Kress W, Tücke J, Mortier W (2003) X-linked myotubular myopathy in a female infant caused by a new MTM1 gene mutation. Neurology 60:1363–1365
Shapiro MB, Senapathy P (1987) RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15:7155–7174
Sutton IJ, Winer JB, Norman AN, Liechti-Gallati S, MacDonald F (2001) Limb girdle and facial weakness in female carriers of X-linked myotubular myopathy mutations. Neurology 57:900–902
Yu S, Manson S, White S et al (2003) X-linked myotubular myopathy in a family with three adult survivors. Clin Genet 64:148–152
Acknowledgments
We are grateful to Nigel Clarke, MD, PhD, for his helpful advice; Michael Walls, PhD, for critical reading of the manuscript; Isabelle Marty, PhD, for providing anti-triadin antibodies; Andrée Rouche, MSc, Svetlana Maugenre, BSc, Bernard Prudhon, BSc, and Nicolas Dondaine, BSc, for expert technical assistance. This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Association Française contre les Myopathies (AFM). J. A. Bevilacqua was supported by the Program Alban; The European Union Program of High Level Scholarships for Latin America (Scholarship No. E04E028343CL); and the Association Institut de Myologie (AIM), France.
Conflict of interest statement
The authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bevilacqua, J.A., Bitoun, M., Biancalana, V. et al. “Necklace” fibers, a new histological marker of late-onset MTM1-related centronuclear myopathy. Acta Neuropathol 117, 283–291 (2009). https://doi.org/10.1007/s00401-008-0472-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0472-1