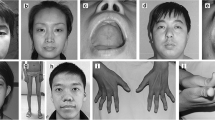
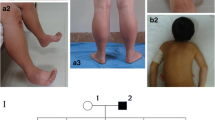
Abstract
Centronuclear myopathy (CNM) is a group of congenital myopathies with the histopathological findings of centralized nuclei in muscle fibres. In this study, we summarized the mutational spectrum and phenotypic features of nine Chinese patients with CNM and reanalysed the existing data on 32 CNM patients reported in China. In a cohort comprising nine patients, 14 variants were found in three CNM-related genes, including DNM2, RYR1, and TTN, in 4, 3, and 2 patients, respectively. Of the total 14 variants identified, nine were reported, and 5 were novel including one pathogenic, one likely pathogenic, and 3 of undetermined significance (VUS). Pathologically, we identified the percentage of muscle fibres with central nuclei was much higher in the DNM2-related CNM patients than that in other genetic type of CNM. Of the 32 genetic-diagnosed CNM patients previously reported from China, DNM2, MTM1, SPEG, RYR1, and MYH7 mutations accounted for 59.4%, 25.0%, 9.4%, 3.1%, and 3.1%, respectively. Notably, all of the 20 variants of DNM2 were missense mutations, and the missense mutations in exon 8 were found in 60.0% of DNM2 variants. The c.1106G > A/ p.R369Q (NM_001005360) occurred in 26.3% patients of this Chinese cohort with DNM2-CNM. In conclusion, CNM showed a highly variable genetic spectrum, with DNM2 as the most common causative gene in Chinese CNM patients.

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
The software and code in this study are available from the corresponding author upon request.
References
Chen T, Pu CQ, Wang Q et al (2015) Clinical, pathological, and genetic features of dynamin-2-related centronuclear myopathy in China. Neurol Sci 36(5):735–741. https://doi.org/10.1007/s10072-014-2028-6
Jungbluth H, Wallgren-Pettersson C, Laporte J (2008) Centronuclear (myotubular) myopathy. Orphanet J Rare Dis 3:26. https://doi.org/10.1186/1750-1172-3-26
Bitoun M, Maugenre S, Jeannet PY et al (2005) Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat Genet 37(11):1207–1209. https://doi.org/10.1038/ng1657
Romero NB, Bitoun M (2011) Centronuclear myopathies. Semin Pediatr Neurol 18(4):250–256. https://doi.org/10.1016/j.spen.2011.10.006
Romero NB (2010) Centronuclear myopathies: a widening concept. Neuromuscul Disord 20(4):223–228. https://doi.org/10.1016/j.nmd.2010.01.014
Pierson CR, Kinga T, Pankaj A et al (2005) X-linked myotubular and centronuclear myopathies. J Neuropathol Exp Neurol 64(7):555–564. https://doi.org/10.1097/01.jnen.0000171653.17213.2e
McEntagart M, Parsons G, Buj-Bello A et al (2002) Genotype-phenotype correlations in X-linked myotubular myopathy. Neuromuscul Disord 12(10):939–946. https://doi.org/10.1016/s0960-8966(02)00153-0
Jungbluth H, Gautel M (2014) Pathogenic mechanisms in centronuclear myopathies. Front Aging Neurosci 6:339. https://doi.org/10.3389/fnagi.2014.00339
Oliveira J, Oliveira ME, Kress W (2013) Expanding the MTM1 mutational spectrum: novel variants including the first multi-exonic duplication and development of a locus-specific database. Eur J Hum Genet 21:540–549. https://doi.org/10.1038/ejhg.2012.201
Biancalana V, Scheidecker S, Miguet M et al (2017) Affected female carriers of MTM1 mutations display a wide spectrum of clinical and pathological involvement: delineating diagnostic clues. Acta Neuropathol 134(6):889–904. https://doi.org/10.1007/s00401-017-1748-0
Majczenko K, Davidson A, Camelo-Piragua S et al (2012) Dominant mutation of CCDC78 in a unique congenital myopathy with prominent internal nuclei and atypical cores. Am J Hum Genet 91(2):365–371. https://doi.org/10.1016/j.ajhg.2012.06.012
Böhm J, Biancalana V, Malfatti E et al (2014) Adult-onset autosomal dominant centronuclear myopathy due to BIN1 mutations. Brain 137(12):3160–3170. https://doi.org/10.1093/brain/awu272
Wilmshurst JM, Lillis S, Zhou H, Pillay K, Henderson H et al (2010) RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol 68(5):717–726. https://doi.org/10.1002/ana.22119
Agrawal PB, Pierson CR, Joshi M et al (2014) SPEG interacts with myotubularin, and its deficiency causes centronuclear myopathy with dilated cardiomyopathy. Am J Hum Genet 95(2):218–226. https://doi.org/10.1016/j.ajhg.2014.07.004
Yu M, Zhu Y, Xie ZY et al (2019) Novel TTN mutations and muscle imaging characteristics in congenital titinopathy. Ann Clin Transl Neurol 6(7):1311–1318. https://doi.org/10.1002/acn3.50831
Bitoun M, Bevilacqua JA, Prudhon B et al (2007) Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol 62(6):666–670. https://doi.org/10.1002/ana.21235
Tammaro A, Bracco A, Cozzolino S et al (2007) Scanning for mutations of the ryanodine receptor (RYR1) gene by denaturing HPLC: detection of three novel malignant hyperthermia alleles. Clin Chem 49(5):761–768. https://doi.org/10.1373/49.5.761
Sato I, Wu S, Ibarra MCA et al (2008) Congenital neuromuscular disease with uniform type 1 fiber and RYR1 mutation. Neurology 70(2):114–122. https://doi.org/10.1212/01.wnl.0000269792.63927.86
Lin PF, Liu XH, Zhao DD et al (2016) DNM2 mutations in Chinese Han patients with centronuclear myopathy. Neurol Sci 37(6):995–998. https://doi.org/10.1007/s10072-016-2513-1
Xiao H, Liu XY, Chen Lu et al (2020) A case of de novo dynamin 2 (DNM2)-related centronuclear myopathy with electrical but not clinical myotonia. Chin Med J (Engl) 133(24):3023–3024. https://doi.org/10.1097/CM9.0000000000000974
Liu X, Wu HM, Niu FB et al (2015) The clinical diagnosis characteristics of DNM2-related centronuclear myopathy and literature review. Chin J Clin (Electronic Edition) 9(13):2477–2481
Zhang YQ, Gu WH, Hao Y et al (2007) Clinical, pathogenic and molecular biological study on a family of autosomal dominant inherited centronuclear myopathy. Chin J Neurol 40:10
Zhao Y, Zhao Z, Shen HR et al (2018) Characterization and genetic diagnosis of centronuclear myopathies in seven Chinese patients. Neurol Sci 39(12):2043–2051. https://doi.org/10.1007/s10072-018-3534-8
Zheng S, Liu XJ, Tian XY (2021) Two cases of neonatal centronuclear myopathy caused by MTM1 mutation. J Med Inform 34:6. https://doi.org/10.3969/j.issn.1006-1959.2021.06.054
Geng HZ, Li W, Yan CZ et al (2020) Clinical, muscle pathological and genetic analyses of a centronuclear myopathy pedigree with a mutation in MTM1 gene. J Shandong Univ (Health Sciences) 58:6
Li N, Zhao Z, Shen H et al (2018) MYH7 mutation associated with two phenotypes of myopathy. Neurol Sci 39(2):333–339. https://doi.org/10.1007/s10072-017-3192-2
Tang J, Ma W, Chen YR et al (2020) Novel SPEG variant cause centronuclear myopathy in China. J Clin Lab Anal 34(2):e23054. https://doi.org/10.1002/jcla.23054
Zhang G, Xu M, Huang T et al (2021) Clinical and genetic analysis of a case with centronuclear myopathy caused by SPEG gene mutation: a case report and literature review. BMC Pediatr 21:209. https://doi.org/10.1186/s12887-021-02656-6
Koutsopoulos OS, Kretz C, Weller CM et al (2013) Dynamin 2 homozygous mutation in humans with a lethal congenital syndrome. Eur J Hum Genet 21(6):637–642. https://doi.org/10.1038/ejhg.2012.226
Böhm J, Biancalana V, Dechene ET et al (2012) Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum Mutat 33(6):949–959. https://doi.org/10.1002/humu.22067
Abath NO, de Araújo C, Moreno M, Malfatti E et al (2017) Common and variable clinical, histological, and imaging findings of recessive RYR1-related centronuclear myopathy patients. Neuromuscul Disord 27(11):975–985. https://doi.org/10.1016/j.nmd.2017.05.016
Tammaro A, Bracco A, Cozzolino S et al (2003) Scanning for mutations of the ryanodine receptor (RYR1) gene by denaturing HPLC: detection of three novel malignant hyperthermia alleles. Clin Chem 49(5):761–768. https://doi.org/10.1373/49.5.761
Dubowitz V, Sewry CA (2021) Muscle biopsy-A practical approach. Elsevier, Philadelphia
Lawal TA, Todd JJ, Meilleur KG (2018) Ryanodine receptor 1-related myopathies: diagnostic and therapeutic approaches. Neurotherapeutics 15(4):885–889. https://doi.org/10.1007/s13311-018-00677-1
Jungbluth H, Zhou HY, Sewry CA et al (2007) Centronuclear myopathy due to a de novo dominant mutation in the skeletal muscle ryanodine receptor (RYR1) gene. Neuromuscul Disord 17(4):338–345. https://doi.org/10.1016/j.nmd.2007.01.016
Ceyhan-Birsoy O, Agrawal PB, Carlos Hidalgo O et al (2013) Recessive truncating titin gene, TTN, mutations presenting as centronuclear myopathy. Neurology 81(14):1205–1214. https://doi.org/10.1212/wnl.0b013e3182a6ca62
Fattori F, Maggi L, Bruno C et al (2015) Centronuclear myopathies: genotype-phenotype correlation and frequency of defined genetic forms in an Italian cohort. J Neurol 262(7):1728–1740. https://doi.org/10.1007/s00415-015-7757-9
Hanisch F, Müller T, Dietz A et al (2011) Phenotype variability and histopathological findings in centronuclear myopathy due to DNM2 mutations. J Neurol 258(6):1085–1090. https://doi.org/10.1007/s00415-010-5889-5
Mikell CB, Chan AK, Stein GE et al (2013) Muscle and nerve biopsies: techniques for the neurologist and neurosurgeon. Clin Neurol Neurosurg 115(8):1206–1214. https://doi.org/10.1016/j.clineuro.2013.05.008
Acknowledgements
The authors acknowledge all patients and their families for participating in this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Zhaoxia Wang take full responsibility for the paper. Material preparation, data collection, and analysis were performed by Qi Wang, Meng Yu, Jing Liu, and Qingqing Wang. Zhiying Xie checked all the genetic analyses. The first draft of the manuscript was written by Qi Wang and Meng Yu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Peking University First Hospital (2021[061]).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publishing their data and photographs.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Table S1.
The 700 genes included in the neuromuscular disease panel. (docx 30.7 kb)
Rights and permissions
About this article
Cite this article
Wang, Q., Yu, M., Xie, Z. et al. Mutational and clinical spectrum of centronuclear myopathy in 9 cases and a literature review of Chinese patients. Neurol Sci 43, 2803–2811 (2022). https://doi.org/10.1007/s10072-021-05627-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05627-y