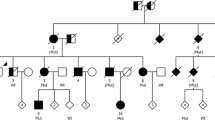
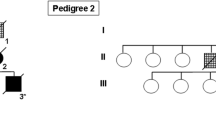
Abstract
Microduplications of the 17q21.31 chromosomal region encompassing the MAPT gene, which encodes the Tau protein, were identified in patients with a progressive disorder initially characterized by severe memory impairment with or without behavioral changes that can clinically mimic Alzheimer disease. The unique neuropathological report showed a primary tauopathy, which could not be unanimously classified in a given known subtype, showing both 4R- and 3R-tau inclusions, mainly within temporal cortical subregions and basal ganglia, without amyloid deposits. Recently, two subjects harboring the same duplication were reported with an atypical extrapyramidal syndrome and gait disorder. To decipher the phenotypic spectrum associated with MAPT duplications, we studied ten carriers from nine families, including two novel unrelated probands, gathering clinical (n = 10), cerebrospinal fluid (n = 6), MRI (n = 8), dopamine transporter scan (n = 4), functional (n = 5), amyloid (n = 3) and Tau-tracer (n = 2) PET imaging data as well as neuropathological examination (n = 4). Ages at onset ranged from 37 to 57 years, with prominent episodic memory impairment in 8/10 patients, associated with behavioral changes in four, while two patients showed atypical extrapyramidal syndrome with gait disorder at presentation, including one with associated cognitive deficits. Amyloid imaging was negative but Tau imaging showed significant deposits mainly in both mesiotemporal cortex. Dopaminergic denervation was found in 4/4 patients, including three without extrapyramidal symptoms. Neuropathological examination exclusively showed Tau-immunoreactive lesions. Distribution, aspect and 4R/3R tau aggregates composition suggested a spectrum from predominantly 3R, mainly cortical deposits well correlating with cognitive and behavioral changes, to predominantly 4R deposits, mainly in the basal ganglia and midbrain, in patients with prominent extrapyramidal syndrome. Finally, we performed in vitro seeding experiments in HEK-biosensor cells. Morphological features of aggregates induced by homogenates of three MAPT duplication carriers showed dense/granular ratios graduating between those induced by homogenates of a Pick disease and a progressive supranuclear palsy cases. These results suggest that MAPT duplication causes a primary tauopathy associated with diverse clinical and neuropathological features.





Similar content being viewed by others
References
Alexander J, Kalev O, Mehrabian S, Traykov L, Raycheva M, Kanakis D et al (2016) Familial early-onset dementia with complex neuropathologic phenotype and genomic background. Neurobiol Aging 42:199–204
Bronner IF, ter Meulen BC, Azmani A, Severijnen LA, Willemsen R, Kamphorst W et al (2005) Hereditary Pick’s disease with the G272V tau mutation shows predominant three-repeat tau pathology. Brain 128:2645–2653
Caffrey TM, Joachim C, Paracchini S, Esiri MM, Wade-Martins R (2006) Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet 15:3529–3537
Chen Z, Chen JA, Shatunov A, Jones AR, Kravitz SN, Huang AY et al (2019) Genome-wide survey of copy number variants finds MAPT duplications in progressive supranuclear palsy. Mov Dis 34:1049–1059
Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A et al (2009) Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 11:909–913
Dan A, Takahashi M, Masuda-Suzukake M, Kametani F, Nonaka T, Kondo H et al (2013) Extensive deamidation at asparagine residue 279 accounts for weak immunoreactivity of tau with RD4 antibody in Alzheimer’s disease brain. Acta Neuropathol Commun 1:54
Daude N, Kim C, Kang S-G, Eskandari-Sedighi G, Haldiman T, Yang J et al (2020) Diverse, evolving conformer populations drive distinct phenotypes in frontotemporal lobar degeneration caused by the same MAPT-P301L mutation. Acta Neuropathol 139:1045–1070
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K et al (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13:614–629
Furman JL, Holmes BB, Diamond MI (2015) Sensitive Detection of Proteopathic Seeding Activity with FRET Flow Cytometry. J Vis Exp. https://doi.org/10.3791/53205
Godefroy O, Azouvi P, Robert P, Roussel M, LeGall D, Meulemans T et al (2010) Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol 68:855–864
Holmes BB, Furman JL, Mahan TE, Yamasaki TR, Mirbaha H, Eades WC et al (2014) Proteopathic tau seeding predicts tauopathy in vivo. PNAS 111:E4376–E4385
Hooli BV, Kovacs-Vajna ZM, Mullin K, Blumenthal MA, Mattheisen M, Zhang C et al (2014) Rare autosomal copy number variations in early-onset familial Alzheimer’s disease. Molecular Psy 19:676–681
Ingelsson M, Ramasamy K, Russ C, Freeman SH, Orne J, Raju S et al (2007) Increase in the relative expression of tau with four microtubule binding repeat regions in frontotemporal lobar degeneration and progressive supranuclear palsy brains. Acta Neuropathol 114:471–479
Kovacs GG (2015) Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41:3–23
Le Guennec K, Quenez O, Nicolas G, Wallon D, Rousseau S, Richard A-C et al (2017) 17q21.31 duplication causes prominent tau-related dementia with increased MAPT expression. Molecular Psy 22:1119–1125
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A et al (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG et al (2015) Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 78:787–800
Myers AJ, Pittman AM, Zhao AS, Rohrer K, Kaleem M, Marlowe L et al (2007) The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis 25:561–570
Neumann M, Schulz-Schaeffer W, Crowther RA, Smith MJ, Spillantini MG, Goedert M et al (2001) Pick’s disease associated with the novel Tau gene mutation K369I. Ann Neurol 50:503–513
Rösler TW, Marvian AT, Brendel M, Nykänen N-P, Höllerhage M, Schwarz SC et al (2019) Four-repeat tauopathies. Prog Neurobiol 180:101644
Rovelet-Lecrux A, Hannequin D, Guillin O, Legallic S, Jurici S, Wallon D et al (2010) Frontotemporal dementia phenotype associated with MAPT gene duplication. JAD 21:897–902
Sahara N, Kimura T (2018) Biochemical properties of pathology-related tau species in tauopathy brains: an extraction protocol for tau oligomers and aggregates. Methods Mol Biol 1779:435–445
Sarazin M, Berr C, Rotrou JD, Fabrigoule C, Pasquier F, Legrain S et al (2007) Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology 69:1859–1867
Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P et al (1993) A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 114:7–12
Sierra M, Martínez-Rodríguez I, Sánchez-Juan P, González-Aramburu I, Jiménez-Alonso M, Sánchez-Rodríguez A et al (2017) Prospective clinical and DaT-SPECT imaging in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 89:439–444
Tacik P, DeTure MA, Carlomagno Y, Lin W, Murray ME, Baker MC et al (2017) FTDP-17 with Pick body-like inclusions associated with a novel tau mutation, p. E372G. Brain Pathol 27:612–626
Thierry M, Boluda S, Delatour B, Marty S, Seilhean D, Network BN-CN et al (2020) Human subiculo-fornico-mamillary system in Alzheimer’s disease: Tau seeding by the pillar of the fornix. Acta Neuropathol 139:443–461
Trabzuni D, Wray S, Vandrovcova J, Ramasamy A, Walker R, Smith C et al (2012) MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet 21:4094–4103
Uchihara T, Kondo H, Ikeda K, Kosaka K (1995) Alzheimer-type pathology in melanin-bleached sections of substantia nigra. J Neurol 242:485–489
Yamamoto T, Hirano A (1986) A comparative study of modified Bielschowsky, bodian and thioflavin S stains on Alzheimer’s neurofibrillary tangles. Neuropath Appl Neuro 12:3–9
Acknowledgements
This work was supported by Fondation pour la Recherche Médicale (Equipe FRM DEQ20170336711). Two of the neuropathological cases described in this paper (ALZ_441_005 and ALZ_596_006) have been collected by the Neuro-CEB brain bank, Paris, France. We thank Luc Buée for helpful advices and Gabor Kovacs for sharing unpublished data. We thank the technical team at Raymond Escourolle neuropathology department. We thank the ICM-Quant core facility, Paris Brain Institute (ICM). Two patients were included in the Shatau7-Imatau study (NTC025768210). MS, JL and MB acknowledge the chemical/radiopharmaceutical and nursing staff of the Service Hospitalier Frederic Joliot for the synthesis of [11C]-PIB and [18F]-AV-1451 and patient management. MS, JL and MB are also indebted to AVID Radiopharmaceuticals, Inc., for their support in supplying the AV-1451 precursor and chemistry production advice. This study was cofunded by the French Ministry of Health grant (PHRC-2013-0919), CEA, Fondation pour la recherche sur Alzheimer, Institut de recherche internationale Servier, France-Alzheimer.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have nothing to disclose in relation to this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
401_2021_2320_MOESM1_ESM.tiff
Supplementary Fig. 1. Morphological variability at macroscopic examination between the four postmortem studied cases: ALZ_441_005 (a and b), ALZ_595_006 (c), EXT_1198_001 (d), EXT_2001_001 (e). a. Marked atrophy of the frontal, parietal and temporal association cortices. b. Coronal sections at two levels, the head of the caudate and the mamillary bodies, showed marked dilatation of the lateral ventricle, severe atrophy of the striatum (white arrow) and of the temporal lobe (black arrow). The hippocampus and amygdala are barely identifiable. c. Moderate atrophy of the medial temporal lobe (red arrow) associated to a moderate dilatation of the temporal horn of the lateral ventricle. d and e. Absence of brain atrophy. Moderate dilatation of the 3rd ventricle in EXT_2000_001 (e). (a and b) left hemisphere; (c) right hemisphere) (TIFF 20381 KB)
401_2021_2320_MOESM2_ESM.tiff
Supplementary Fig. 2. Diversity of tau pathology in different regions of the brain. In the pallidum, tau pathology was abundant in all cases except in ALZ_596_006. In the substantia nigra tau aggregates where numerous in all cases seen where tissue was available. Globular tau aggregates in neuronal cell body and neuropil threads were the predominating deposits. In the pons, the basis pontis showed a great number of tau positive aggregates which were globular in ALZ_441_005. Tau deposits were scant in EXT_2000_001 and were absent in ALZ_596_006 and EXT_1998_001. The dentate nucleus showed abundant coarse granular neuronal intracytoplasmic aggregates in ALZ_441_005, deposits were moderate in EXT_2000_001 and scant in the other two cases. The white matter showed little pathology in ALZ_596_006 and EXT_2000_001 with few neuropil threads. In ALZ_441_005 a moderate number of oligodendrocytic aggregates were seen and were predominantly globular. In EXT_1998_001, the few oligodendroglial pathology seen adopted a more typical coiled body shape. Immunohistochemical staining with AT8. Scale bar in rows 1-4: 50µm, Scale bar in last row: 25µm (TIFF 9220 KB)
401_2021_2320_MOESM3_ESM.tiff
Supplementary Fig. 3. a. Immunohistochemical and histochemical characteristics of tau aggregates. Tau aggregates seen in neurons and astrocytes in the four histological studied cases (ALZ_441_005, ALZ_596_006, EXT_1998_001, EXT_2000_001) were positive for phosphorylation dependent AT100 antibody, p62 and Gallyas silver staining. Occasional neurons and rarely astrocytes in the frontal cortex of case ALZ_596_006 showed argyrophilic properties with the Bielschowsky silver staining. Frontal cortex (ALZ_441_005 and ALZ_596_006) and striatum (EXT_1998_001 and EXT_2000_001). Immunohistochemistry: AT100 and p62. Silver staining: Gallyas and Bielschowsky. Scale bar 50µm except for Bielschowsy staining of case ALZ_596_006 which is 25µm. b. White matter pathology is scant to moderate in ALZ_441_005. Tau aggregates are seen in some of the oligodendrocytes. There is a predominance of small round aggregates (black arrow) but some coiled bodies are also seen (white arrow head). Tau aggregates express the 3R-tau and 4R-tau isoforms, are AT100 and p62 positive and are argyrophilic with both Gallyas and Bielschowsky silver stains. Immunohistochemistry: AT8, 3R-tau, 4R-tau, AT100 and p62. Silver staining: Gallyas and Bielschowsky. Scale bar for top left image 100µm, for top right image 50µm and for inferior row 25µm. c. Only rare neurons in the dentate gyrus and CA2 region of the hippocampus are positive for Bielschowsky in ALZ_441_005. Bielschowsky silver stain. Scale bar: 25µm. d. Round neuronal intracytoplasmic tau aggregates were observed in the dentate gyrus in ALZ_596_006. The aggregates were immunoreactive for both 3R-tau and 4R-tau antibodies and were positive for Gallyas and Bielschowsky silver staining. Immunohistochemistry: 3R-tau and 4R-tau. Silver stains: Gallyas and Bielschowsky. Scale bar: 25µm. e. Perivascular thorny astrocytes reminiscent of aging-related tau astrogliopathy (ARTAG) were seen in the periamygdaloid region. Additionally, there were occasional astrocytes containing tau aggregates in the subcortical white matter (parietal lobe). Immunohistochemistry: AT8. Scale bar: 50µm. f. In case ALZ_596_006, 3R tau and 4R tau neurofibrillary tangles and neuropil threads in the hippocampus-CA1. Immunohistochemistry: 3R-tau, 4R-tau. Silver staining: Gallyas and Bielschowsky. Scale bar: 50 µm (TIFF 7107 KB)
401_2021_2320_MOESM4_ESM.tiff
Supplementary Fig. 4. Western blot using WO2 antibody from the frontal cortex of three MAPT duplication carriers, a subject with Alzheimer disease due to an APP duplication, and one normal control (TIFF 10263 KB)
401_2021_2320_MOESM5_ESM.tiff
Supplementary Fig. 5. Analysis of the 4R/3R ratio of MAPT mRNA. Extracted from frontal cortex or striatum. The control group (ctrls) consists in the mean of five normal individuals. Error bars represent SEM. ALZ 441: ALZ_441_005; ALZ 596: ALZ_596_006, EXT 1998: EXT_1998_001 (TIFF 7134 KB)
401_2021_2320_MOESM6_ESM.tiff
Supplementary Fig. 6. Quantitative analysis of tau seeding activity in a cell biosensor. Proportion of HEK cells containing endogenous tau intracellular aggregates for each tested sample, obtained after 2 x 4 repeated observations in three independent experiments. Patients were compared into a linear mixed effect model for repeated data using Tukey correction for multiple comparisons (Mean of observed proportions ± SEM: CTRL: 0.22% ± 0.11%, PiD cortex: 2.90% ± 0.51%; ALZ_441_005 striatum: 12.22 ± 0.81; ALZ_596_006: 4.48 ± 0.61% ; EXT_1998_001 striatum: 5.95% ± 0.83%; PSP : 14.93% ± 0.90% ). Proportion of cells with aggregates is significantly higher in all patients compared to controls (PiD vs CTRL: p=0.01; other comparison to controls: all p<0.001). Tau seeding activities detected in patients were all pairwise significantly different (ALZ_441_005 vs PSP: p=0.03, EXT_1998_001 vs PiD: p = 0.01, all other p<0.001) except for PiD vs ALZ_596_006 (p=0.6) and EXT_1998_001 vs ALZ_596_006 (p=0.6) (TIFF 6775 KB)
401_2021_2320_MOESM7_ESM.tiff
Supplementary Fig 7. Absence of associated ⍺-synucleinopathy or TDP-43-pathy. Immunohistochemistry for ⍺-synuclein and TDP-43 was negative in all histological studied cases. Aβ was negative in three cases (ALZ_441_005, EXT_1998_001, EXT_2000_001). A few diffuse plaques were seen in the neocortex of ALZ_596_006. First column: frontal cortex. Second column: substantia nigra (SN). Third column: dentate gyrus. Scale bar: 50 µm (TIFF 12293 KB)
401_2021_2320_MOESM8_ESM.docx
Supplementary Table 1. Staining properties of tau immunoreactive aggregates in different cell types in MAPT duplication carriers. 3R-tau, 4R-tau, AT100 and p62 antibodies. Gallyas and Bielschowsky silver staining. R: tau aggregates are sparsely positive. ND: not determined. (DOCX 18 kb)
Rights and permissions
About this article
Cite this article
Wallon, D., Boluda, S., Rovelet-Lecrux, A. et al. Clinical and neuropathological diversity of tauopathy in MAPT duplication carriers. Acta Neuropathol 142, 259–278 (2021). https://doi.org/10.1007/s00401-021-02320-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-021-02320-4