Abstract
Parkinson disease (PD) is a neurodegenerative disorder characterized by massive loss of midbrain dopaminergic neurons. Whereas onset of motor impairments reflects a rather advanced stage of the disorder, hyposmia often marks the beginning of the disease. Little is known about the role of the nigro-striatal system in olfaction under physiological conditions and the anatomical basis of hyposmia in PD. Yet, the early occurrence of olfactory dysfunction implies that pathogens such as environmental toxins could incite the disease via the olfactory system. In the present study, we demonstrate a dopaminergic innervation from neurons in the substantia nigra to the olfactory bulb by axonal tracing studies. Injection of two dopaminergic neurotoxins—1-methyl-4-phenylpyridinium and 6-hydroxydopamine—into the olfactory bulb induced a decrease in the number of dopaminergic neurons in the substantia nigra. In turn, ablation of the nigral projection led to impaired olfactory perception. Hyposmia following dopaminergic deafferentation was reversed by treatment with the D1/D2/D3 dopamine receptor agonist rotigotine. Hence, we demonstrate for the first time the existence of a direct dopaminergic projection into the olfactory bulb and identify its origin in the substantia nigra in rats. These observations may provide a neuroanatomical basis for invasion of environmental toxins into the basal ganglia and for hyposmia as frequent symptom in PD.






Similar content being viewed by others
References
Ansari KA, Johnson A (1975) Olfactory function in patients with Parkinson’s disease. J Chronic Dis 28:493–497
Attems J, Walker L, Jellinger KA (2014) Olfactory bulb involvement in neurodegenerative diseases. Acta Neurolpathol 127:459–475
Beach TG, Iii CLW, Hladik CL, Adler CH (2009) Olfactory bulb α-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neurolpathol 117:169–174. doi:10.1007/s00401-008-0450-7.Olfactory
Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, Wolters EC (2001) Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol 50:34–41
Van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, van de Berg WDJ, Hol EM (2011) The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain 134:3249–3263. doi:10.1093/brain/awr256
Björklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202. doi:10.1016/j.tins.2007.03.006
Borta A, Höglinger GU (2007) Dopamine and adult neurogenesis. J Neurochem 100:587–595. doi:10.1111/j.1471-4159.2006.04241.x
Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Coronas V, Srivastava LK, Liang JJ, Jourdan F, Moyse E (1997) Identification and localization of dopamine receptor subtypes in rat olfactory mucosa and bulb: a combined in situ hybridization and ligand binding radioautographic approach. J Chem Neuroanat 12:243–257
Dauer WT, Przedborski S (2003) Parkinson’ s disease: mechanisms and Models. Neuron 39:889–909
Davis BJ, Macrides F, Youngs WM, Schneider SP, Rosene DL (1978) Efferent and centrifugal afferents of the main and accessory olfactory bulbs in the hamster. Brain Res Bull 3:59–72
Doty RL (2012) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8:329–339. doi:10.1038/nrneurol.2012.80
Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI (1992) Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 55:138–142
Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S (2004) Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci 24:8354–8365. doi:10.1523/JNEUROSCI.2751-04.2004
Escanilla O, Yuhas C, Marzan D, Linster C (2009) Dopaminergic modulation of olfactory bulb processing affects odor discrimination learning in rats. Behav Neurosci 123:828–833. doi:10.1037/a0015855
Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet M-F (2008) Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci 28:247–256. doi:10.1111/j.1460-9568.2008.06346.x
Freundlieb N, François C, Tandé D, Oertel WH, Hirsch EC, Höglinger GU (2006) Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci 26:2321–2325. doi:10.1523/JNEUROSCI.4859-05.2006
Frielingsdorf H, Schwarz K, Brundin P, Mohapel P (2004) No evidence for new dopaminergic neurons in the adult mammalian substantia nigra. Proc Natl Acad Sci USA 101:10177–10182. doi:10.1073/pnas.0401229101
Gaillard I, Rouquier S, Giorgi D (2004) Olfactory receptors. Cell Mol Life Sci 61:456–469. doi:10.1007/s00018-003-3273-7
Gutièrrez-Mecinas M, Crespo C, Blasco-Ibáñez JM, Gracia-Llanes FJ, Marqués-Marí AI, Nácher J, Varea E, Martínez-Guijarro FJ (2005) Distribution of D2 dopamine receptor in the olfactory glomeruli of the rat olfactory bulb. Eur J Neurosci 22:1357–1367. doi:10.1111/j.1460-9568.2005.04328.x
Höglinger GU, Arias-Carrión O, Ipach B, Oertel WH (2014) Origin of the dopaminergic innervation of adult neurogenic areas. J Comp Neurol 522:2336–2348. doi:10.1002/cne.23537
Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC (2004) Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735. doi:10.1038/nn1265
Huisman E, Uylings HBM, Hoogland PV (2004) A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord 19:687–692. doi:10.1002/mds.10713
Huisman E, Uylings HBM, Hoogland PV (2008) Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson’s disease patients. Mov Disord 23:1407–1413. doi:10.1002/mds.22009
Van Kampen JM, Hagg T, Robertson HA (2004) Induction of neurogenesis in the adult rat subventricular zone and neostriatum following dopamine D3 receptor stimulation. Eur J Neurosci 19:2377–2387. doi:10.1111/j.0953-816X.2004.03342.x
Kehr J, Hu X-J, Goiny M, Scheller DKA (2007) Continuous delivery of rotigotine decreases extracellular dopamine suggesting continuous receptor stimulation. J Neural Transm 114:1027–1031. doi:10.1007/s00702-007-0719-3
Kim Y, Wang W-Z, Comte I, Pastrana E, Tran PB, Brown J, Miller RJ, Doetsch F, Molnár Z, Szele FG (2010) Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem 114:750–760. doi:10.1111/j.1471-4159.2010.06799.x
Koster NL, Norman AB, Richtand NM, Nickell WT, Puche AC, Pixley SK, Shipley MT (1999) Olfactory receptor neurons express D2 dopamine receptors. J Comp Neurol 411:666–673
Laska M, Shepherd GM (2007) Olfactory discrimination ability of CD-1 mice for a large array of enantiomers. Neuroscience 144:295–301. doi:10.1016/j.neuroscience.2006.08.063
Lazarini F, Lledo P-M (2011) Is adult neurogenesis essential for olfaction? Trends Neurosci 34:20–30. doi:10.1016/j.tins.2010.09.006
Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ (1993) Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA 90:8861–8865
Lévy F, Meurisse M, Ferreira G, Thibault J, Tillet Y (1999) Afferents to the rostral olfactory bulb in sheep with special emphasis on the cholinergic, noradrenergic and serotonergic connections. J Chem Neuroanat 16:245–263
Lledo P-M, Gheusi G, Vincent J-D (2005) Information processing in the mammalian olfactory system. Physiol Rev 85:281–317. doi:10.1152/physrev.00008.2004
Lledo P-M, Merkle FT, Alvarez-Buylla A (2008) Origin and function of olfactory bulb interneuron diversity. Trends Neurosci 31:392–400. doi:10.1016/j.tins.2008.05.006
Mendoza-Torreblanca JG, Martínez-Martínez E, Tapia-Rodríguez M, Ramírez-Hernández R, Gutiérrez-Ospina G (2008) The rostral migratory stream is a neurogenic niche that predominantly engenders periglomerular cells: in vivo evidence in the adult rat brain. Neurosci Res 60:289–299. doi:10.1016/j.neures.2007.11.013
Merkle FT, Mirzadeh Z, Alvarez-Buylla A (2007) Mosaic organization of neural stem cells in the adult brain. Science 317:381–384. doi:10.1126/science.1144914
Motles E, Tetas M, Gomez A (1995) Behavioral effects evoked by SKF 38393 and LY 171555 in adult cats. Physiol Behav 57:983–988
Mundiñano I-C, Hernandez M, DiCaudo C, Ordoñez C, Marcilla I, Tuñon M-T, Luquin M-R (2013) Reduced cholinergic olfactory centrifugal inputs in patients with neurodegenerative disorders and MPTP-treated monkeys. Acta Neurolpathol 126:411–425
O’Keeffe GC, Barker RA, Caldwell MA (2009) Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle 8:2888–2894
O’Keeffe GC, Tyers P, Aarsland D, Dalley JW, Barker RA, Caldwell MA (2009) Dopamine-induced proliferation of adult neural precursor cells in the mammalian subventricular zone is mediated through EGF. Proc Natl Acad Sci USA 106:8754–8759. doi:10.1073/pnas.0803955106
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, Sydney
Pignatelli A, Kobayashi K, Okano H, Belluzzi O (2005) Functional properties of dopaminergic neurones in the mouse olfactory bulb. J Physiol 564:501–514. doi:10.1113/jphysiol.2005.084632
Ponsen MM, Stoffers D, Booij J, van Eck-Smit BLF, Wolters EC, Berendse HW (2004) Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol 56:173–181. doi:10.1002/ana.20160
Prediger RDS, Aguiar AS, Moreira ELG, Matheus FC, Castro AA, Walz R, De Bem AF, Latini A, Tasca CI, Farina M, Raisman-Vozari R (2011) The intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a new rodent model to test palliative and neuroprotective agents for Parkinson’s disease. Curr Pharm Des 17:489–507
Rey NL, Petit GH, Bousset L, Melki R, Brundin P (2013) Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathol 126:555–573. doi:10.1007/s00401-013-1160-3
Schwarting RK, Huston JP (1996) The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 50:275–331
Shepherd GM (2004) The human sense of smell: are we better than we think? PLoS Biol 2:E146. doi:10.1371/journal.pbio.0020146
Shipley MT, Adamek GD (1984) The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull 12:669–688
Shipley MT, Ennis M (1996) Functional organization of olfactory system. J Neurobiol 30:123–176. doi:10.1002/(SICI)1097-4695(199605)30:1<123:AID-NEU11>3.0.CO;2-N
Slotnick B, Cockerham R, Pickett E (2004) Olfaction in olfactory bulbectomized rats. J Neurosci 24:9195–9200. doi:10.1523/JNEUROSCI.1936-04.2004
Soh Z, Saito M, Kurita Y, Takiguchi N, Ohtake H, Tsuji T (2014) A comparison between the human sense of smell and neural activity in the olfactory bulb of rats. Chem Senses 39:91–105. doi:10.1093/chemse/bjt057
Stiasny-Kolster K, Doerr Y, Möller JC, Höffken H, Behr TM, Oertel WH, Mayer G (2005) Combination of “idiopathic” REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 128:126–137. doi:10.1093/brain/awh322
Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW (2006) Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res 172:97–105. doi:10.1016/j.bbr.2006.04.025
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493
Valle-Leija P, Drucker-Colín R (2014) Unilateral olfactory deficit in a hemiparkinson’s disease mouse model. NeuroReport 25:948–953. doi:10.1097/WNR.0000000000000218
Wei CJ, Linster C, Cleland TA (2006) Dopamine D(2) receptor activation modulates perceived odor intensity. Behav Neurosci 120:393–400. doi:10.1037/0735-7044.120.2.393
Winner B, Desplats P, Hagl C, Klucken J, Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, Buerger E, Winkler J (2009) Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp Neurol 219:543–552. doi:10.1016/j.expneurol.2009.07.013
Winner B, Geyer M, Couillard-Despres S, Aigner R, Bogdahn U, Aigner L, Kuhn G, Winkler J (2006) Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol 197:113–121. doi:10.1016/j.expneurol.2005.08.028
Woulfe JM, Gray MT, Gray DA, Munoz DG, Middeldorp JM (2014) Hypothesis: a role for EBV-induced molecular mimicry in Parkinson’s disease. Parkinsonism Relat Disord 20:685–694. doi:10.1016/j.parkreldis.2014.02.031
Zhang S, Xiao Q, Le W (2015) Olfactory Dysfunction and Neurotransmitter Disturbance in Olfactory Bulb of Transgenic Mice Expressing Human A53T Mutant α-Synuclein. PLoS One 10:e0119928. doi:10.1371/journal.pone.0119928
Acknowledgments
This work was supported by the German Federal Ministry of Education and Research Network “Stem Cells in PD” (Grant 01GN0513), the Deutsche Forschungsgemeinschaft (DFG; HO2402/6-2 to GUH), the German Academic Exchange Service (DAAD to OAC), intramural funds of the University of Lübeck (to DAF), UCB Pharma S.A., the Förderverein Neurologie Marburg and the Charitable Hertie Foundation, Frankfurt/Main, Germany (to WHO).
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. U. Höglinger, D. Alvarez-Fischer and O. Arias-Carrión contributed equally to the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2015_1451_MOESM1_ESM.pptx
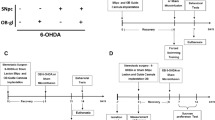
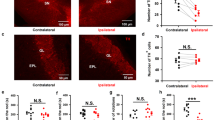
Supplementary Figure S1 | Axonal projection of the substantia nigra pars compacta into the olfactory bulb (Anterograde tracing). (a) Sagittal section of a rat brain immunostained for tyrosine hydroxylase (TH, brown) shows the location of the dopaminergic neurons of the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) in the midbrain, as well as their axonal projections via the medial forebrain bundle (MFB) to the striatum (Str), nucleus accumbens (NA) and olfactory tubercle (OT). The periphery of the olfactory bulb (OB) also contains dopaminergic neurons. The lines indicate the level of the photographs shown at higher magnifications in coronal sections in the panels (b-e). (b) Confocal microscopic image showing as overview and at higher magnification of the boxed area that the tracer substance DiI (red) was injected precisely into SNc (TH+ neurons in green) and that there was no local diffusion to surrounding tissues. (c) Confocal microscopic image with 3D-reconstruction showing the SVZ, delineated by the dashed lines, located between the Str and the lateral ventricle (LV), which contains numerous newborn BrdU+ neuroblasts (blue) and TH+ fibers (green, green arrows), some of which were DiI+ (red, red arrows) after injection of the tracer into the SNc. The crossed lines depict a TH+ DiI+ fiber in the lower left quadrant; colocalization of both markers is verified in the x–z and y–z axis. (d) Confocal microscopic image of the OB, using NeuN immunostaining (red) to delineate the anatomical regions, shown as overview and at higher magnification of the boxed area, demonstrating TH+ (green) neurons in the glomerular layer (GL) and TH+ fibers in the external plexiform layer (EPL), mitral cell layer (MCL) and granular cell layer (GCL). Internal plexiform layer (IPL) (e) Confocal microscopic image from the OB at high magnification, showing TH+ (green) fibers containing DiI (red) in the EPL, the GCL, and mainly in the MCL, but not in the GL, after injection of the DiI tracer into the SNc. Scale bars: 400 µm (a); 100 µm (b-e); 50 µm (insert in d). (PPTX 9998 kb)
401_2015_1451_MOESM2_ESM.pptx
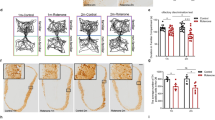
Supplementary Figure S2 | Rotigotine stimulates precursor cell proliferation in the subventricular zone. (a) The efficacy of the 6-OHDA lesion was demonstrated by a marked reduction in TH+ fibers in the striatum ipsilateral to the 6-OHDA injection. Staining of adjacent sections for BrdU demonstrated reduced numbers of newborn BrdU+ neuroblasts in the SVZ of the 6-OHDA-lesioned side compared to the unlesioned control side. (b) Amphetamine-induced net rotational scores ipsiversive to the side of the 6-OHDA lesion demonstrate functionally effective lesions of the nigro-striatal dopaminergic pathway in all groups; there were no significant differences in the degree of rotations between the experimental groups; the rotigotine doses given in the x-axis represent future group assignment, i.e. no rotigotine treatment was given prior to or during this examination. (c) Optical density measures of the TH-immunostained striatum showed a reduction in dopaminergic fibers on the lesioned side compared to the contralateral control side by about 70 % in all experimental groups (*p < 0.05 lesioned vs. control side, two-sided t-test), without significant differences in the degree of lesion between groups. (d) Effect of rotigotine administered in different concentrations for 14 days on the BrdU+ cell number in the SVZ. Black: unlesioned control side. White: 6-OHDA-lesioned side. * P < 0.05 vs. unlesioned side, 0 mg rotigotine; # P < 0.01 vs. lesioned side, 0 mg rotigotine; ANOVA, post hoc LSD test. (e) Reduction in the number of BrdU+ cells in the SVZ on the lesioned side, expressed as % of the control side. * p < 0.05 vs. lesioned vs. unlesioned side, same rotigotine-dose; ANOVA, post hoc-LSD test. (PPTX 1239 kb)
Rights and permissions
About this article
Cite this article
Höglinger, G.U., Alvarez-Fischer, D., Arias-Carrión, O. et al. A new dopaminergic nigro-olfactory projection. Acta Neuropathol 130, 333–348 (2015). https://doi.org/10.1007/s00401-015-1451-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1451-y