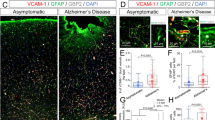
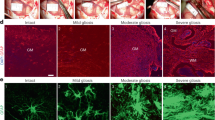
Abstract
Multiple sclerosis (MS) lesions are characterized by the presence of activated astrocytes, which are thought to actively take part in propagating lesion progression by secreting pro-inflammatory mediators. Conversely, reactive astrocytes may exert disease-dampening effects through the production of trophic factors and anti-inflammatory mediators. Astrocytic control of the blood–brain barrier (BBB) is crucial for normal brain homeostasis and BBB disruption is a well-established early event in MS lesion development. Here, we set out to unravel potential protective effects of reactive astrocytes on BBB function under neuroinflammatory conditions as seen in MS, where we focus on the role of the brain morphogen retinoic acid (RA). Immunohistochemical analysis revealed that retinaldehyde dehydrogenase 2 (RALDH2), a key enzyme for RA synthesis, is highly expressed by reactive astrocytes throughout white matter lesions compared to control and normal appearing white matter. In vitro modeling of reactive astrocytes resulted in increased expression of RALDH2, enhanced RA synthesis, and a protective role for astrocyte-derived RA on BBB function during inflammation-induced barrier loss. Furthermore, RA induces endothelial immune quiescence and decreases monocyte adhesion under inflammatory conditions. Finally, we demonstrated that RA attenuated oxidative stress in inflamed endothelial cells, through activation of the antioxidant transcription factor nuclear factor E2 related factor 2. In summary, RA synthesis by reactive astrocytes represents an endogenous protective response to neuroinflammation, possibly aimed at protecting the BBB against inflammatory insult. A better understanding of RA signaling in MS pathophysiology may lead to the discovery of novel targets to halt disease progression.




Similar content being viewed by others
References
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci 7:41–53
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A (2011) The Hedgehog pathway promotes blood–brain barrier integrity and CNS immune quiescence. Science 334:1727–1731
Becher B, Giacomini PS, Pelletier D, McCrea E, Prat A, Antel JP (1999) Interferon-gamma secretion by peripheral blood T-cell subsets in multiple sclerosis: correlation with disease phase and interferon-beta therapy. Ann Neurol 45:247–250
Bitsch A, Kuhlmann T, Da CC, Bunkowski S, Polak T, Bruck W (2000) Tumour necrosis factor alpha mRNA expression in early multiple sclerosis lesions: correlation with demyelinating activity and oligodendrocyte pathology. Glia 29:366–375
Bruck W, Sommermeier N, Bergmann M, Zettl U, Goebel HH, Kretzschmar HA, Lassmann H (1996) Macrophages in multiple sclerosis. Immunobiology 195:588–600
Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM (2006) Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53:688–695
Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308
De Groot CJ, Langeveld CH, Jongenelen CA, Montagne L, van der Valk P, Dijkstra CD (1997) Establishment of human adult astrocyte cultures derived from postmortem multiple sclerosis and control brain and spinal cord regions: immunophenotypical and functional characterization. J Neurosci Res 49:342–354
Duester G (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134:921–931
Elkord E, Williams PE, Kynaston H, Rowbottom AW (2005) Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology 114:204–212
Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A (1998) IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol 28:2178–2187
Fabis MJ, Scott GS, Kean RB, Koprowski H, Hooper DC (2007) Loss of blood–brain barrier integrity in the spinal cord is common to experimental allergic encephalomyelitis in knockout mouse models. Proc Natl Acad Sci USA 104:5656–5661
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155
Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H (2012) NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135:886–899
Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ (2008) SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol Heart Circ Physiol 294:H891–H900
Garcia-Vallejo JJ, Van Het Hof B, Robben J, Van Wijk JA, Van Die I, Joziasse DH, Van Dijk W (2004) Approach for defining endogenous reference genes in gene expression experiments. Anal Biochem 329:293–299
Ge S, Shrestha B, Paul D, Keating C, Cone R, Guglielmotti A, Pachter JS (2012) The CCL2 synthesis inhibitor bindarit targets cells of the neurovascular unit, and suppresses experimental autoimmune encephalomyelitis. J Neuroinflammation 9:171
Haroon F, Drogemuller K, Handel U, Brunn A, Reinhold D, Nishanth G, Mueller W, Trautwein C, Ernst M, Deckert M, Schluter D (2011) Gp130-dependent astrocytic survival is critical for the control of autoimmune central nervous system inflammation. J Immunol 186:6521–6531
Hindinger C, Bergmann CC, Hinton DR, Phares TW, Parra GI, Hussain S, Savarin C, Atkinson RD, Stohlman SA (2012) IFN-gamma signaling to astrocytes protects from autoimmune mediated neurological disability. PLoS One 7:e42088
Huang JK, Jarjour AA, Nait OB, Kerninon C, Williams A, Krezel W, Kagechika H, Bauer J, Zhao C, Baron-Van EA, Chambon P, Ffrench-Constant C, Franklin RJ (2011) Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci 14:45–53
Katsuki H, Kurimoto E, Takemori S, Kurauchi Y, Hisatsune A, Isohama Y, Izumi Y, Kume T, Shudo K, Akaike A (2009) Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J Neurochem 110:707–718
Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H (2007) A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 315:820–825
Kim JH, Yu KS, Jeong JH, Lee NS, Lee JH, Jeong YG, Yoo YC, Han SY (2013) All-trans-retinoic acid rescues neurons after global ischemia by attenuating neuroinflammatory reactions. Neurochem Res 38:2604–2615
Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Hörsten S, Shudo K, Oki S, Yamamura T (2009) Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am J Pathol 174:2234–2245
Konig R, Stillfried M, Aperdannier P, Clarner T, Beyer C, Kipp M, Mey J (2012) Expression of retinoid X receptor beta is induced in astrocytes during corpus callosum demyelination. J Chem Neuroanat 43:120–132
Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, het van Hof B, Scheffer G, Scheper R, Dijkstra CD, van der Valk P, de Vries HE (2011) Adenosine triphosphate-binding cassette transporters mediate chemokine (C–C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 134:555–570
Kornyei Z, Gocza E, Ruhl R, Orsolits B, Voros E, Szabo B, Vagovits B, Madarasz E (2007) Astroglia-derived retinoic acid is a key factor in glia-induced neurogenesis. FASEB J 21:2496–2509
Kort JJ, Kawamura K, Fugger L, Weissert R, Forsthuber TG (2006) Efficient presentation of myelin oligodendrocyte glycoprotein peptides but not protein by astrocytes from HLA-DR2 and HLA-DR4 transgenic mice. J Neuroimmunol 173:23–34
Lassmann H, Bruck W, Lucchinetti C (2001) Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol Med 7:115–121
Lassmann H, van Horssen J (2011) The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett 585:3715–3723
Lassmann H, van Horssen J, Mahad D (2012) Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 8:647–656
Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481
Loken-Amsrud KI, Myhr KM, Bakke SJ, Beiske AG, Bjerve KS, Bjornara BT, Hovdal H, Lilleas F, Midgard R, Pedersen T, Benth JS, Torkildsen O, Wergeland S, Holmoy T (2013) Retinol levels are associated with magnetic resonance imaging outcomes in multiple sclerosis. Mult Scler 19:451–457
Lucchinetti C, Bruck W, Noseworthy J (2001) Multiple sclerosis: recent developments in neuropathology, pathogenesis, magnetic resonance imaging studies and treatment. Curr Opin Neurol 14:259–269
Mendes A, Sa MJ (2011) Classical immunomodulatory therapy in multiple sclerosis: how it acts, how it works. Arq Neuropsiquiatr 69:536–543
Minagar A, Alexander JS (2003) Blood–brain barrier disruption in multiple sclerosis. Mult Scler 9:540–549
Mizee MR, Wooldrik D, Lakeman KA, van het Hof B, Drexhage JA, Geerts D, Bugiani M, Aronica E, Mebius RE, Prat A, de Vries HE, Reijerkerk A (2013) Retinoic acid induces blood–brain barrier development. J Neurosci 33:1660–1671
Moehlenkamp JD, Johnson JA (1999) Activation of antioxidant/electrophile-responsive elements in IMR-32 human neuroblastoma cells. Arch Biochem Biophys 363:98–106
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65:2702–2720
Paul D, Ge S, Lemire Y, Jellison ER, Serwanski DR, Ruddle NH, Pachter JS (2014) Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J Neuroinflammation 11:10
Puttagunta R, Di GS (2011) Retinoic acid signaling in axonal regeneration. Front Mol Neurosci 4:59
Reijerkerk A, Kooij G, van der Pol SM, Khazen S, Dijkstra CD, de Vries HE (2006) Diapedesis of monocytes is associated with MMP-mediated occludin disappearance in brain endothelial cells. FASEB J 20:2550–2552
Rice GP, Hartung HP, Calabresi PA (2005) Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology 64:1336–1342
Rudick R, Polman C, Clifford D, Miller D, Steinman L (2013) Natalizumab: bench to bedside and beyond. JAMA Neurol 70:172–182
Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L (2006) Side by side comparison between dynamic versus static models of blood–brain barrier in vitro: a permeability study. Brain Res 1109:1–13
Schreibelt G, Kooij G, Reijerkerk A, van Doorn R, Gringhuis SI, van der Pol S, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE (2007) Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21:3666–3676
Schreibelt G, van Horssen J, Haseloff RF, Reijerkerk A, van der Pol SM, Nieuwenhuizen O, Krause E, Blasig IE, Dijkstra CD, Ronken E, de Vries HE (2008) Protective effects of peroxiredoxin-1 at the injured blood-brain barrier. Free Radic Biol Med 45:256–264
Shearer KD, Fragoso YD, Clagett-Dame M, McCaffery PJ (2012) Astrocytes as a regulated source of retinoic acid for the brain. Glia 60:1964–1976
Speth C, Dierich MP, Sopper S (2005) HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol Immunol 42:213–228
Tani M, Glabinski AR, Tuohy VK, Stoler MH, Estes ML, Ransohoff RM (1996) In situ hybridization analysis of glial fibrillary acidic protein mRNA reveals evidence of biphasic astrocyte activation during acute experimental autoimmune encephalomyelitis. Am J Pathol 148:889–896
Van der Goes A, Wouters D, van der Pol SM, Huizinga R, Ronken E, Adamson P, Greenwood J, Dijkstra CD, de Vries HE, de Vries HE (2001) Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J 15:1852–1854
van Doorn R, Nijland PG, Dekker N, Witte ME, Lopes-Pinheiro MA, Van Het Hof B, Kooij G, Kooij G, Reijerkerk A, Dijkstra C, van der Valk P, van Horssen J, de Vries HE (2012) Fingolimod attenuates ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes. Acta Neuropathol 124:397–410
van Horssen J, Witte ME, Schreibelt G, de Vries HE (2011) Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta 1812:141–150
Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29:11511–11522
Wang C, Napoli JL (2011) Multiple retinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes. J Biol Chem 286:6542–6553
Wang XJ, Hayes JD, Henderson CJ, Wolf CR (2007) Identification of retinoic acid as an inhibitor of transcription factor Nrf2 through activation of retinoic acid receptor alpha. Proc Natl Acad Sci USA 104:19589–19594
Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO (2005) Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J 19:1872–1874
Weston AD, Chandraratna RA, Torchia J, Underhill TM (2002) Requirement for RAR-mediated gene repression in skeletal progenitor differentiation. J Cell Biol 158:39–51
Witte ME, Nijland PG, Drexhage JA, Gerritsen W, Geerts D, Van Het Hof B, Reijerkerk A, de Vries HE, van der Valk P, van Horssen J (2013) Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol 125:231–243
Wong LF, Yip PK, Battaglia A, Grist J, Corcoran J, Maden M, Azzouz M, Kingsman SM, Kingsman AJ, Mazarakis ND, McMahon SB (2006) Retinoic acid receptor beta2 promotes functional regeneration of sensory axons in the spinal cord. Nat Neurosci 9:243–250
Xu J, Drew PD (2006) 9-cis-retinoic acid suppresses inflammatory responses of microglia and astrocytes. J Neuroimmunol 171:135–144
Zhang T, Liang X, Shi L, Wang L, Chen J, Kang C, Zhu J, Mi M (2013) Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(−)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS ONE 8:e79075
Zhao F, Wu T, Lau A, Jiang T, Huang Z, Wang XJ, Chen W, Wong PK, Zhang DD (2009) Nrf2 promotes neuronal cell differentiation. Free Radic Biol Med 47:867–879
Acknowledgments
This work was supported by Dutch Foundation of MS Research Grants MS 07-615 and MS 12-797 (M.M.) and MS 09-358 (PN). The authors declare that they have no conflict of interest. We would like to thank W.N. van Wieringen (Dept. Epidemiology and Biostatistics, VU University medical center, Amsterdam) for statistical support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mizee, M.R., Nijland, P.G., van der Pol, S.M.A. et al. Astrocyte-derived retinoic acid: a novel regulator of blood–brain barrier function in multiple sclerosis. Acta Neuropathol 128, 691–703 (2014). https://doi.org/10.1007/s00401-014-1335-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1335-6