Abstract
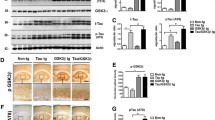
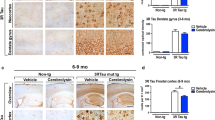
Alzheimer’s disease (AD) continues to be the most common cause of cognitive and motor alterations in the aging population. Accumulation of amyloid β (Aβ)-protein oligomers and the microtubule associated protein-TAU might be responsible for the neurological damage. We have previously shown that Cerebrolysin (CBL) reduces the synaptic and behavioral deficits in amyloid precursor protein (APP) transgenic (tg) mice by decreasing APP phosphorylation via modulation of glycogen synthase kinase-3β (GSK3β) and cyclin-dependent kinase-5 (CDK5) activity. These kinases also regulate TAU phosphorylation and are involved in promoting neurofibrillary pathology. In order to investigate the neuroprotective effects of CBL on TAU pathology, a new model for neurofibrillary alterations was developed using somatic gene transfer with adeno-associated virus (AAV2)-mutant (mut) TAU (P301L). The Thy1-APP tg mice (3 m/o) received bilateral injections of AAV2-mutTAU or AAV2-GFP, into the hippocampus. After 3 months, compared to non-tg controls, in APP tg mice intra-hippocampal injections with AAV2-mutTAU resulted in localized increased accumulation of phosphorylated TAU and neurodegeneration. Compared with vehicle controls, treatment with CBL in APP tg injected with AAV2-mutTAU resulted in a significant decrease in the levels of TAU phosphorylation at critical sites dependent on GSK3β and CDK5 activity. This was accompanied by amelioration of the neurodegenerative alterations in the hippocampus. This study supports the concept that the neuroprotective effects of CBL may involve the reduction of TAU phosphorylation by regulating kinase activity.








Similar content being viewed by others
References
Alvarez XA, Cacabelos R, Laredo M, Couceiro V, Sampedro C, Varela M, Corzo L, Fernandez-Novoa L, Vargas M, Aleixandre M, Linares C, Granizo E, Muresanu D, Moessler H (2006) A 24-week, double-blind, placebo-controlled study of three dosages of Cerebrolysin in patients with mild to moderate Alzheimer’s disease. Eur J Neurol 13:43–54. doi:10.1111/j.1468-1331.2006.01222.x
Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86:582–590. doi:10.1046/j.1471-4159.2003.01879.x
Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM (2007) Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 27:9115–9129. doi:10.1523/JNEUROSCI.2361-07.2007
Brion JP, Tremp G, Octave JN (1999) Transgenic expression of the shortest human tau affects its compartmentalization and its phosphorylation as in the pretangle stage of Alzheimer’s disease. Am J Pathol 154:255–270
Caccamo A, Oddo S, Tran LX, LaFerla FM (2007) Lithium reduces tau phosphorylation but not A beta or working memory deficits in a transgenic model with both plaques and tangles. Am J Pathol 170:1669–1675. doi:10.2353/ajpath.2007.061178
Chana G, Landau S, Beasley C, Everall IP, Cotter D (2003) Two-dimensional assessment of cytoarchitecture in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia: evidence for decreased neuronal somal size and increased neuronal density. Biol Psychiatry 53:1086–1098. doi:10.1016/S0006-3223(03)00114-8
Chana G, Everall IP, Crews L, Langford D, Adame A, Grant I, Cherner M, Lazzaretto D, Heaton R, Ellis R, Masliah E (2006) Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology 67:1486–1489. doi:10.1212/01.wnl.0000240066.02404.e6
Cho JH, Johnson GV (2003) Glycogen synthase kinase 3beta phosphorylates tau at both primed and unprimed sites. Differential impact on microtubule binding. J Biol Chem 278:187–193. doi:10.1074/jbc.M206236200
Cho JH, Johnson GV (2004) Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau’s ability to bind and stabilize microtubules. J Neurochem 88:349–358
Davies P, Ghanbari H, Issacs A, Dickson D, Mattiace L, Rosado M, Vincent I (1993) TG3: a better antibody that Alz-50 for the visualization of Alzheimer-type neuronal pathology. Soc Neurosci Abstr 19:1636
DeKosky S, Scheff S (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 27:457–464. doi:10.1002/ana.410270502
Delobel P, Lavenir I, Fraser G, Ingram E, Holzer M, Ghetti B, Spillantini MG, Crowther RA, Goedert M (2008) Analysis of tau phosphorylation and truncation in a mouse model of human tauopathy. Am J Pathol 172:123–131. doi:10.2353/ajpath.2008.070627
Duff K (2001) Transgenic mouse models of Alzheimer’s disease: phenotype and mechanisms of pathogenesis. Biochem Soc Symp 67:195–202
Flaherty DB, Soria JP, Tomasiewicz HG, Wood JG (2000) Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res 62:463–472. doi:10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7
Francis-Turner L, Valouskova V (1996) Nerve growth factor and nootropic drug Cerebrolysin but not fibroblast growth factor can reduce spatial memory impairment elicited by fimbria-fornix transection: short-term study. Neurosci Lett 202:1–4. doi:10.1016/0304-3940(95)12240-0
Goedert M, Hasegawa M (1999) The tauopathies: toward an experimental animal model. Am J Pathol 154:1–6
Gomez-Ramos A, Dominguez J, Zafra D, Corominola H, Gomis R, Guinovart JJ, Avila J (2006) Inhibition of GSK3 dependent tau phosphorylation by metals. Curr Alzheimer Res 3:123–127. doi:10.2174/156720506776383059
Gotz J, Probst A, Spillantini MG, Schafer T, Jakes R, Burki K, Goedert M (1995) Somatodendritic localization and hyperphosphorylation of tau protein in transgenic mice expressing the longest human brain tau isoform. EMBO J 14:1304–1313
Gotz J, Chen F, Barmettler R, Nitsch RM (2001) Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem 276:529–534. doi:10.1074/jbc.M006531200
Gotz J, Chen F, van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293:1491–1495. doi:10.1126/science.1062097
Hashimoto M, Sagara Y, Everall IP, Mallory M, Everson A, Langford D, Masliah E (2002) Fibroblast growth factor 1 regulates signaling via the GSK3β pathway: implications for neuroprotection. J Biol Chem 277:32985–32991. doi:10.1074/jbc.M202803200
Hong M, Chen DC, Klein PS, Lee VM (1997) Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem 272:25326–25332. doi:10.1074/jbc.272.40.25326
Hutton M, Lewis J, Dickson D, Yen S, McGowan E (2001) Analysis of tauopathies with transgenic mice. Trends Mol Med 7:467–470. doi:10.1016/S1471-4914(01)02123-2
Hyman B, Gomez-Isla T (1994) Alzheimer’s disease is a laminar regional and neural system specific disease, not a global brain disease. Neurobiol Aging 15:353–354. doi:10.1016/0197-4580(94)90031-0
Hyman BT, Augustinack JC, Ingelsson M (2005) Transcriptional and conformational changes of the tau molecule in Alzheimer’s disease. Biochim Biophys Acta 1739:150–157
Illenberger S, Zheng-Fischhofer Q, Preuss U, Stamer K, Baumann K, Trinczek B, Biernat J, Godemann R, Mandelkow EM, Mandelkow E (1998) The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer’s disease. Mol Biol Cell 9:1495–1512
Jicha GA, Bowser R, Kazam IG, Davies P (1997) Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res 48:128–132. doi:10.1002/(SICI)1097-4547(19970415)48:2<128::AID-JNR5>3.0.CO;2-E
Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R (2003) APP processing and synaptic function. Neuron 37:925–937. doi:10.1016/S0896-6273(03)00124-7
Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM (2006) Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther 13:517–527. doi:10.1016/j.ymthe.2005.10.008
Koo E, Lansbury PJ, Kelly J (1999) Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc Natl Acad Sci USA 96:9989–9990. doi:10.1073/pnas.96.18.9989
Lee HG, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA (2005) Tau phosphorylation in Alzheimer’s disease: pathogen or protector? Trends Mol Med 11:164–169. doi:10.1016/j.molmed.2005.02.008
Lee VM (1996) Regulation of tau phosphorylation in Alzheimer’s disease. Ann N Y Acad Sci 777:107–113. doi:10.1111/j.1749-6632.1996.tb34408.x
Lee VM, Trojanowski JQ (1999) Neurodegenerative tauopathies: human disease and transgenic mouse models. Neuron 24:507–510. doi:10.1016/S0896-6273(00)81106-X
Lee VM, Goedert M, Trojanowski JQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24:1121–1159. doi:10.1146/annurev.neuro.24.1.1121
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491. doi:10.1126/science.1058189
Li T, Hawkes C, Qureshi HY, Kar S, Paudel HK (2006) Cyclin-dependent protein kinase 5 primes microtubule-associated protein tau site-specifically for glycogen synthase kinase 3beta. Biochemistry 45:3134–3145. doi:10.1021/bi051635j
Li T, Paudel HK (2006) Glycogen synthase kinase 3beta phosphorylates Alzheimer’s disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism. Biochemistry 45:3125–3133. doi:10.1021/bi051634r
Mallory M, Honer W, Hsu L, Johnson R, Masliah E (1999) In vitro synaptotrophic effects of Cerebrolysin in NT2N cells. Acta Neuropathol 97:437–446. doi:10.1007/s004010051012
Mandelkow EM, Biernat J, Drewes G, Gustke N, Trinczek B, Mandelkow E (1995) Tau domains, phosphorylation, and interactions with microtubules. Neurobiol Aging 16:355–362. doi:10.1016/0197-4580(95)00025-A discussion 362–353
Mandelkow EM, Schweers O, Drewes G, Biernat J, Gustke N, Trinczek B, Mandelkow E (1996) Structure, microtubule interactions, and phosphorylation of tau protein. Ann N Y Acad Sci 777:96–106. doi:10.1111/j.1749-6632.1996.tb34407.x
Masliah E (1995) Mechanisms of synaptic dysfunction in Alzheimer’s disease. Histol Histopathol 10:509–519
Masliah E, Armasolo F, Veinbergs I, Mallory M, Samuel W (1999) Cerebrolysin ameliorates performance deficits and neuronal damage in apolipoprotein E-deficient mice. Pharmacol Biochem Behav 62:239–245
Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287:1265–1269. doi:10.1126/science.287.5456.1265
McCown TJ (2005) Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther 5:333–338. doi:10.2174/1566523054064995
Mi K, Johnson GV (2006) The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 3:449–463. doi:10.2174/156720506779025279
Michel G, Mercken M, Murayama M, Noguchi K, Ishiguro K, Imahori K, Takashima A (1998) Characterization of tau phosphorylation in glycogen synthase kinase-3beta and cyclin dependent kinase-5 activator (p23) transfected cells. Biochim Biophys Acta 1380:177–182
Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L (2000) High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci 20:4050–4058
Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA 102:6990–6995. doi:10.1073/pnas.0500466102
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421. doi:10.1016/S0896-6273(03)00434-3
Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM (2004) Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43:321–332. doi:10.1016/j.neuron.2004.07.003
Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM (2006) Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem 281:39413–39423. doi:10.1074/jbc.M608485200
Paxinos G, Franklin K (2003) The mouse brain in stereotaxic coordinates. Academic Press, NY
Peel AL, Klein RL (2000) Adeno-associated virus vectors: activity and applications in the CNS. J Neurosci Methods 98:95–104. doi:10.1016/S0165-0270(00)00183-7
Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J (2003) Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis 5:301–308
Perez M, Ribe E, Rubio A, Lim F, Moran MA, Ramos PG, Ferrer I, Isla MT, Avila J (2005) Characterization of a double (amyloid precursor protein-tau) transgenic: tau phosphorylation and aggregation. Neuroscience 130:339–347. doi:10.1016/j.neuroscience.2004.09.029
Poorkaj P, Grossman M, Steinbart E, Payami H, Sadovnick A, Nochlin D, Tabira T, Trojanowski JQ, Borson S, Galasko D, Reich S, Quinn B, Schellenberg G, Bird TD (2001) Frequency of tau gene mutations in familial and sporadic cases of non-Alzheimer dementia. Arch Neurol 58:383–387. doi:10.1001/archneur.58.3.383
Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH (2000) Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem 74:1587–1595. doi:10.1046/j.1471-4159.2000.0741587.x
Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science 316:750–754. doi:10.1126/science.1141736
Rockenstein E, McConlogue L, Tan H, Power M, Masliah E, Mucke L (1995) Levels and alternative splicing of amyloid β protein precursor (APP) transcripts in brains of APP transgenic mice and humans with Alzheimer’s disease. J Biol Chem 270:28257–28267. doi:10.1074/jbc.270.47.28257
Rockenstein E, Mallory M, Mante M, Sisk A, Masliah E (2001) Early formation of mature amyloid-b proteins deposits in a mutant APP transgenic model depends on levels of Ab1–42. J Neurosci Res 66:573–582. doi:10.1002/jnr.1247
Rockenstein E, Mallory M, Mante M, Alford M, Windisch M, Moessler H, Masliah E (2002) Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer’s disease. J Neural Transm Suppl 62:327–336
Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E (2003) The neuroprotective effects of Cerebrolysin trade mark in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance. J Neural Transm 110:1313–1327. doi:10.1007/s00702-003-0025-7
Rockenstein E, Adame A, Mante M, Larrea G, Crews L, Windisch M, Moessler H, Masliah E (2005) Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer’s disease with the neurotrophic compound cerebrolysin. J Neural Transm 112:269–282. doi:10.1007/s00702-004-0181-4
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E (2006) Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. J Neurosci Res 83(7):1252–1261
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E (2006) Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. J Neurosci Res 83:1252–1261. doi:10.1002/jnr.20818
Rockenstein E, Mante M, Adame A, Crews L, Moessler H, Masliah E (2007) Effects of Cerebrolysin trade mark on neurogenesis in an APP transgenic model of Alzheimer’s disease. Acta Neuropathol 113:265–275. doi:10.1007/s00401-006-0166-5
Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, Crews L, Masliah E (2007) Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci 27:1981–1991. doi:10.1523/JNEUROSCI.4321-06.2007
Ruther E, Ritter R, Apecechea M, Freitag S, Windisch M (1994) Efficacy of Cerebrolysin in Alzheimer’s disease. In: Jellinger K, Ladurner G, Windisch M (eds) New trends in the diagnosis and therapy of Alzheimer’s disease. Springer, Vienna, pp 131–141
Ruther E, Ritter R, Apecechea M, Freytag S, Windisch M (1994) Efficacy of the peptidergic nootropic drug cerebrolysin in patients with senile dementia of the Alzheimer’s type (SDAT). Pharmacopsychiatry 27:32–40. doi:10.1055/s-2007-1014271
Ryder J, Su Y, Liu F, Li B, Zhou Y, Ni B (2003) Divergent roles of GSK3 and CDK5 in APP processing. Biochem Biophys Res Commun 312:922–929. doi:10.1016/j.bbrc.2003.11.014
Schubert D, Heinemann S, Carlisle W, Tarikas H, Kimes B, Patrick J, Steinbach JH, Culp W, Brandt BL (1974) Clonal cell lines from the rat central nervous system. Nature 249:224–227. doi:10.1038/249224a0
Selenica ML, Jensen HS, Larsen AK, Pedersen ML, Helboe L, Leist M, Lotharius J (2007) Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol 152:959–979. doi:10.1038/sj.bjp.0707471
Selkoe DJ, Schenk D (2003) Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol 43:545–584. doi:10.1146/annurev.pharmtox.43.100901.140248
Selkoe DJ (2008) Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res 192:106–113. doi:10.1016/j.bbr.2008.02.016
Sigurdsson EM (2008) Immunotherapy targeting pathological tau protein in Alzheimer’s disease and related tauopathies. J Alzheimers Dis 15:157–168
Sinha S, Anderson J, John V, McConlogue L, Basi G, Thorsett E, Schenk D (2000) Recent advances in the understanding of the processing of APP to beta amyloid peptide. Ann N Y Acad Sci 920:206–208
Spencer B, Rockenstein E, Crews L, Marr R, Masliah E (2007) Novel strategies for Alzheimer’s disease treatment. Expert Opin Biol Ther 7:1853–1867. doi:10.1517/14712598.7.12.1853
Takahashi M, Yasutake K, Tomizawa K (1999) Lithium inhibits neurite growth and tau protein kinase I/glycogen synthase kinase-3beta-dependent phosphorylation of juvenile tau in cultured hippocampal neurons. J Neurochem 73:2073–2083
Takashima A (2006) GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 9:309–317
Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke-Iqbal I (2003) The dentate gyrus neurogenesis: a therapeutic target for Alzheimer’s disease. Acta Neuropathol 105:225–232
Terry R, Hansen L, Masliah E (1994) Structural basis of the cognitive alterations in Alzheimer disease. In: Terry R, Katzman R (eds) Alzheimer disease. Raven Press, New York, pp 179–196
Trojanowski J, Schmidt M, Shin R-W, Bramblett G, Rao D, Lee V-Y (1993) Altered Tau and neurofilament proteins in neurodegenerative diseases: diagnostic implications for Alzheimer’s disease and Lewy body dementias. Brain Pathol 3:45–54. doi:10.1111/j.1750-3639.1993.tb00725.x
Trojanowski JQ, Lee VM (1994) Phosphorylation of neuronal cytoskeletal proteins in Alzheimer’s disease and Lewy body dementias. Ann N Y Acad Sci 747:92–109
Veinbergs I, Mante M, Mallory M, Masliah E (2000) Neurotrophic effects of Cerebrolysin in animal models of excitotoxicity. J Neural Transm Suppl 59:273–280
Walsh DM, Selkoe DJ (2007) A beta oligomers—a decade of discovery. J Neurochem 101:1172–1184. doi:10.1111/j.1471-4159.2006.04426.x
Weaver CL, Espinoza M, Kress Y, Davies P (2000) Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiol Aging 21:719–727. doi:10.1016/S0197-4580(00)00157-3
Wei ZH, He QB, Wang H, Su BH, Chen HZ (2007) Meta-analysis: the efficacy of nootropic agent Cerebrolysin in the treatment of Alzheimer’s disease. J Neural Transm 114:629–634. doi:10.1007/s00702-007-0630-y
Wen Y, Yang SH, Liu R, Perez EJ, Brun-Zinkernagel AM, Koulen P, Simpkins JW (2007) Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim Biophys Acta 1772:473–483
Woods YL, Cohen P, Becker W, Jakes R, Goedert M, Wang X, Proud CG (2001) The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J 355:609–615
Acknowledgments
This work was supported by NIH grant AG05131 and by a grant from EBEWE Pharmaceuticals.
Conflict of interest statement
Research relating to this manuscript was funded in part by a grant from EBEWE Pharmaceuticals; the manufacturer of CBL, and a portion of the scientific background to CBL and experimental design was provided by employees of EBEWE Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1 In vitro characterization of pTAU expression in a neuronal cell lineinfected with AAV2-mutTAU
. pTAU immunoreactivity in B103 neuronal cells infected with AAV2-control (a) andAAV2-mutTAU (b). GFP immunoreactivity in B103 neuronal cells infected with AAV2- control (c) and AAV2-mutTAU (d). Western blot analysis (e). Bar = 50 M (TIFF 8132 kb)
Fig. S2 Time course of pTAU expression in APP tg mice injected with AAV2-mutTAU
.pTAU immunoreactivity at 0 (a), 7 (b), 14 (c) and 30 (d) days post injection with AAV2-mutTAU. Analysis in (e). Scale bar = 50 M (TIFF 12335 kb)
Fig. S3 A immunoreactivity in APP tg mice injected with AAV2-mutTAU
.Aβ immunoreactivity in AAV2-mutTAU injected vehicle-treated (a) and CBL-treated (b)APP tg mice. A immunoreactivity in AAV2-GFP injected vehicle-treated (c) and CBL-26 -treated (d) APP tg mice. Quantitative analysis of % area of A immunoreactive neuropilin AAV2-mutTAU and AAV2-GFP injected vehicle- or CBL-treated APP tg mice (i).Scale bar = 50 M. * Indicates significance at p<0.05 (one way ANOVA) (TIFF 14753 kb)
Rights and permissions
About this article
Cite this article
Ubhi, K., Rockenstein, E., Doppler, E. et al. Neurofibrillary and neurodegenerative pathology in APP-transgenic mice injected with AAV2-mutant TAU: neuroprotective effects of Cerebrolysin. Acta Neuropathol 117, 699–712 (2009). https://doi.org/10.1007/s00401-009-0505-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-009-0505-4