Abstract
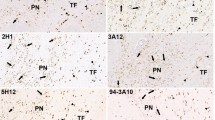
Certain genetic defects in LRRK2 and parkin are pathogenic for Parkinson’s disease (PD) and both proteins deposit in the characteristic Lewy bodies. LRRK2 is thought to be involved in the early initiation of Lewy bodies. The involvement of LRRK2 and parkin in the similar cellular deposition of fibrillar α-synuclein in glial cytoplasmic inclusions (GCI) in multiple system atrophy (MSA) has not yet been assessed. To determine whether LRRK2 and parkin may be similarly associated with the abnormal deposition of α-synuclein in MSA GCI, paraffin-embedded sections from the basal ganglia of 12 patients with MSA, 4 with PD and 4 controls were immunostained for LRRK2, parkin, α-synuclein and oligodendroglial proteins using triple labelling procedures. The severity of neuronal loss was graded and the proportion of abnormally enlarged oligodendroglia containing different combinations of proteins assessed in 80–100 cells per case. Parkin immunoreactivity was observed in only a small proportion of GCI. In contrast, LRRK2 was found in most of the enlarged oligodendroglia in MSA and colocalised with the majority of α-synuclein-immunopositive GCI. Degrading myelin sheaths containing LRRK2-immunoreactivity were also observed, showing an association with one of the earliest oligodendroglial abnormalities observed in MSA. The proportion of LRRK2-immunopositive GCI was negatively associated with an increase in neuronal loss and α-synuclein-immunopositive dystrophic axons. Our results indicate that an increase in LRRK2 expression occurs early in association with myelin degradation and GCI formation, and that a reduction in LRRK2 expression in oligodendroglia is associated with increased neuronal loss in MSA.





Similar content being viewed by others
References
Akaishi T, Shiomi T, Sawada H, Yokosawa H (1996) Purification and properties of the 26S proteasome from the rat brain: evidence for its degradation of myelin basic protein in a ubiquitin-dependent manner. Brain Res 722:139–144. doi:10.1016/0006-8993(96)00212-0
Alegre-Abarrategui J, Ansorge O, Esiri M, Wade-Martins R (2008) LRRK2 is a component of granular alpha-synuclein pathology in the brainstem of Parkinson’s disease. Neuropathol Appl Neurobiol 34:272–283. doi:10.1111/j.1365-2990.2007.00888.x
Baker KG, Huang Y, McCann H et al (2006) P25alpha immunoreactive but alpha-synuclein immunonegative neuronal inclusions in multiple system atrophy. Acta Neuropathol 111:193–195. doi:10.1007/s00401-005-0008-x
Boggs JM (2006) Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 63:1945–1961. doi:10.1007/s00018-006-6094-7
Braak H, Rub U, Del Tredici K (2003) Involvement of precerebellar nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 29:60–76. doi:10.1046/j.1365-2990.2003.00432.x
Campbell BC, McLean CA, Culvenor JG et al (2001) The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem 76:87–96. doi:10.1046/j.1471-4159.2001.00021.x
Gai WP, Power JH, Blumbergs PC, Blessing WW (1998) Multiple-system atrophy: a new alpha-synuclein disease? Lancet 352:547–548. doi:10.1016/S0140-6736(05)79256-4
Gai WP, Pountney DL, Power JH et al (2003) alpha-Synuclein fibrils constitute the central core of oligodendroglial inclusion filaments in multiple system atrophy. Exp Neurol 181:68–78. doi:10.1016/S0014-4886(03)00004-9
Giasson BI, Covy JP, Bonini NM et al (2006) Biochemical and pathological characterization of Lrrk2. Ann Neurol 59:315–322. doi:10.1002/ana.20791
Greggio E, Jain S, Kingsbury A et al (2006) Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis 23:329–341
Higashi S, Biskup S, West AB et al (2007) Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res 1155:208–219. doi:10.1016/j.brainres.2007.04.034
Jaleel M, Nichols RJ, Deak M et al (2007) LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem J 405:307–317. doi:10.1042/BJ20070209
Jellinger KA (2006) P25alpha immunoreactivity in multiple system atrophy and Parkinson disease. Acta Neuropathol 112:112. doi:10.1007/s00401-006-0075-7
Jellinger KA, Seppi K, Wenning GK (2005) Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord 20(Suppl 12):S29–S36. doi:10.1002/mds.20537
Kato S, Nakamura H (1990) Cytoplasmic argyrophilic inclusions in neurons of pontine nuclei in patients with olivopontocerebellar atrophy: immunohistochemical and ultrastructural studies. Acta Neuropathol 79:584–594. doi:10.1007/BF00294235
Kovács GG, Gelpi E, Lehotzky A et al (2007) The brain-specific protein TPPP/p25 in pathological protein deposits of neurodegenerative diseases. Acta Neuropathol 113:153–161. doi:10.1007/s00401-006-0167-4
Lindersson E, Beedholm R, Hojrup P et al (2004) Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem 279:12924–12934. doi:10.1074/jbc.M306390200
Melrose HL, Kent CB, Taylor JP et al (2007) A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience 147:1047–1058. doi:10.1016/j.neuroscience.2007.05.027
Miklossy J, Arai T, Guo JP et al (2006) LRRK2 expression in normal and pathological human brain and in human cell lines. J Neuropathol Exp Neurol 65:953–963. doi:10.1097/01.jnen.0000235121.98052.54
Miklossy J, Qing H, Guo JP et al (2007) Lrrk2 and chronic inflammation are linked to pallido-ponto-nigral degeneration caused by the N279K tau mutation. Acta Neuropathol 114:243–254. doi:10.1007/s00401-007-0230-9
Miller DW, Johnson JM, Solano SM et al (2005) Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm 112:1613–1624. doi:10.1007/s00702-005-0378-1
Nishie M, Mori F, Yoshimoto M, Takahashi H, Wakabayashi K (2004) A quantitative investigation of neuronal cytoplasmic and intranuclear inclusions in the pontine and inferior olivary nuclei in multiple system atrophy. Neuropathol Appl Neurobiol 30:546–554. doi:10.1111/j.1365-2990.2004.00564.x
Ozawa T, Okuizumi K, Ikeuchi T et al (2001) Analysis of the expression level of alpha-synuclein mRNA using postmortem brain samples from pathologically confirmed cases of multiple system atrophy. Acta Neuropathol 102:188–190
Ozawa T, Paviour D, Quinn NP et al (2004) The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain 127:2657–2671. doi:10.1093/brain/awh303
Polymeropoulos MH, Lavedan C, Leroy E et al (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047. doi:10.1126/science.276.5321.2045
Schlehe JS, Lutz AK, Pilsl A et al (2008) Aberrant folding of pathogenic Parkin mutants: aggregation versus degradation. J Biol Chem 283:13771–13779. doi:10.1074/jbc.M707494200
Schlossmacher MG, Frosch MP, Gai WP et al (2002) Parkin localizes to the Lewy bodies of Parkinson disease and dementia with Lewy bodies. Am J Pathol 160:1655–1667
Smith WW, Pei Z, Jiang H et al (2005) Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA 102:18676–18681. doi:10.1073/pnas.0508052102
Song YJ, Lundvig DM, Huang Y et al (2007) p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol 171:1291–1303. doi:10.2353/ajpath.2007.070201
Tan EK, Zhao Y, Skipper L et al (2007) The LRRK2 Gly2385Arg variant is associated with Parkinson’s disease: genetic and functional evidence. Hum Genet 120:857–863. doi:10.1007/s00439-006-0268-0
West AB, Moore DJ, Biskup S et al (2005) Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci USA 102:16842–16847. doi:10.1073/pnas.0507360102
Yazawa I, Giasson BI, Sasaki R et al (2005) Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron 45:847–859. doi:10.1016/j.neuron.2005.01.032
Zarate-Lagunes M, Gu WJ, Blanchard V et al (2001) Parkin immunoreactivity in the brain of human and non-human primates: an immunohistochemical analysis in normal conditions and in Parkinsonian syndromes. J Comp Neurol 432:184–196. doi:10.1002/cne.1096
Zhu X, Babar A, Siedlak SL et al (2006) LRRK2 in Parkinson’s disease and dementia with Lewy bodies. Mol Neurodegener 30:1–17
Zhu X, Siedlak SL, Smith MA et al (2006) LRRK2 protein is a component of Lewy bodies. Ann Neurol 60:617–618. doi:10.1002/ana.20928
Acknowledgments
Human brain tissue samples were received from the Australian Brain Donor Programme Prince of Wales Medical Research Institute Tissue Resource Centre, which is supported by the National Health and Medical Research Council of Australia, Australian Brain Donor Programme approved request #GH060505 to GMH. We are grateful to the brain donors and their families for supporting medical research, and to Prof. Poul Henning Jensen (Denmark) for the p25α antibody. This research in Australia was supported by NHMRC project grant 210186. The Queen Square Brain Bank is supported by the Reta Lila Weston Institute for Neurological Studies, the Progressive Supranuclear Palsy (Europe) Association, the Sarah Matheson Trust and the Alzheimer’s Research Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Y., Song, Y.J.C., Murphy, K. et al. LRRK2 and parkin immunoreactivity in multiple system atrophy inclusions. Acta Neuropathol 116, 639–646 (2008). https://doi.org/10.1007/s00401-008-0446-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0446-3