Abstract
One of the emerging technologies of the recent times harboring nanotechnology to fabricate nanofibers for various biomedical and environmental applications are electrospinning (nanofiber technology). Their relative ease in use, simplicity, functionality and diversity has surpassed the pitfalls encountered with the conventional method of generating fibers. This review aims to provide an overview of electrospinning, principle, methods, feed materials, and applications toward tissue engineering. To begin with, evolution of electrospinning and its typical apparatus have been briefed. Simultaneously, discussion on the production of nanofibers with diversified feed materials such as polymers, small molecules, colloids, and nanoparticles and its transformation into a powerful technology has been dealt with. Further, highlights on the application of nanofibers in tissue engineering and the commercialized products developed using nanofiber technology have been summed up. With this rapidly emerging technology, there would be a great demand pertaining to scalability and environmental challenge toward tissue engineering applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The evolution of nanotechnology in the recent years has provided novel approaches in restructuring materials conferring magnificent physical, chemical, and optochemical properties. Such fine tuning in the properties has given rise to materials of zero-dimensional characteristics that include nanoparticles or quantum dots; one-dimensional nanowires, nanorods, nanofibers, and nanotubes; two-dimensional nanosheets and nanofilms; and three-dimensional forms mostly comprising bundles or dispersions of several nanomaterials [1,2,3,4]. Among various nanomaterials, nanofibers have gained popularity as for its wide application is concerned. The outstanding features that make them unique from other nanomaterials are their high surface area to volume ratio, flexibility, mechanical strength, and high porosity. The materials with such characteristics are preferred as a robust candidate for much advanced applications pertaining to tissue engineering, drug delivery and sensors [5, 6]. In addition, they have also been widely employed in textile industries and in aerospace engineering for reinforced clothing. It is the fiber diameter that determines the performance characteristics, processibility, and practicability of the fibrous structures destined to various applications.
Indeed, these were the results of the pioneering works of Professor Darrell Reneker for the introduction, rediscovery, and popularization of the art of electrospinning [7]. It was by electrospinning that a number of materials such as polymers (synthetic and natural), carbon-based materials, semiconducting materials, and even nanocomposites have been recruited to create nanoscale fibers with superior characteristics [8,9,10,11]. Although there are several other alternatives in the fabrication and generation of nanofibers, electrospinning is the much preferred one owing to its simplicity, versatility and viability [12]. Most importantly, there has been a rapid progress in the fabrication and functional applications of nanofibers in biomedical, clinical, and healthcare settings.
Here, a critical review on the evolution, challenges, and emerging development of nanofiber technology in biomedical field and regenerative medicine has been elaborated. As electrospinning is the most sought after technique for synthesizing specific nanofiber for a wide range of applications including electronics, photonics, environmental treatment, energy generation and storage, a clear description about the timelines, fabrication techniques, and their broad spectrum application in areas encompassing biomedical engineering and healthcare industry have been discussed. Alongside, the current state and future directions augmenting commercialization and implementation have also been highlighted.
The need for fiber-based technology
Tissue engineering represents one of the emerging fields that adopt the principles of bio and chemical engineering in achieving the goal of tissue regeneration. It is for this that the technology utilizes various biomaterials, cells, and growth factors either solely or in combinations to restore, maintain, and improve the functional characteristics. This generally involves fabrication of tissue-like constructs mimicking the functional tissue with structural, topographical, and mechanical properties. Secondly, the construct must facilitate diffusion of nutrients and oxygen and removal of metabolic wastes as well during tissue regeneration. Indeed, there were some fiber-based techniques viz. weaving, knitting, braiding, electrospinning, and direct writing to facilitate the construction of 3D scaffolds and cell laden tissue constructs. Construction of such 3D synthetic frameworks would enable cellular attachment, proliferation, and growth, thus leading to the formation of new tissue. The scope of fabricating scaffolds potentially mimicking the natural human tissue at nanometer scale has drawn much attention owing to their high surface area to volume ratio and typical microporous structure that better suited to achieve desired effects in tissue engineering applications [13].
Fiber-based technology and their implications date back to several millennia where the fibrous structures were woven into textiles and used as clothing and for decoration. Their applications have been engineered and extended toward filtration, composite fabrication, energy systems, and microfluidics. In order to fabricate fibers, there are innumerous approaches such as electrospinning, wetspinning, biospinning, interfacial complexation, microfluidic spinning, and meltspinning. Among them, electrospinning seems to be the most promising and attractive technique for tissue engineering applications. It is this technique that offers a better prospect of controlling the thickness, composition of the nanofibers, and porosity with relatively simple protocol. In addition, electrospinning exhibits certain strengths with respect to scaffold fabrication viz. simplicity, efficiency in controlling the flow rate and voltage, and the scaling-up process. However, there are certain challenges associated with electrospinning such as fabrication of thick 3D complex scaffolds, poor control over high fiber packing density leading to small pore size (~ 10–15 μm), and poor cellular infiltration as a result of pore size. Although living cells were encapsulated in electrospun fibers by configuration of the coaxial needle connected to a syringe pump to facilitate constant flow rate, cells could not be accommodated by the fibers owing to porosity issues. Furthermore, cell encapsulated constructs could not be electrospun owing to the lack of control over the distribution of cells in a given volume. Though the cells can be arranged using high electric fields (~ 1–2 kV cm−1), the fate of viability of the cells for tissue reconstruction needs further investigations [14].
Fabrication of biopolymeric fibers with varied topographical properties arranged in a spatiotemporal fashion at micro and/or nanoscale represents the initial step in a fiber-based engineering strategy. These fibers could be used as carriers for biological moieties and microorganisms depending upon the application. Moreover, the biological and mechanical properties of the fibers fabricated determine the functional aspect of the tissue constructs. One of the main characteristic features of the fiber-based technology in tissue engineering is the surface topology, which plays a pivotal role in directing the growth of the cell inside the 3D spatial arrangement [15].
Electrospinning: state of the art
Electrospinning is one of the most widely employed and established techniques used for the generation of specific fibers at different scales [16]. But before man could attempt constructing fibers, he had been greatly inspired by nature where spiders and silkworms served as an important source for the development of artificial fibers [17]. But an interesting fact is that man has already mastered the art of weaving during 5000 BC as evidenced from the fragments of cotton articles unearthed during archaeological excavation. Notably, silkworm cultivation began only during 2700 BC, and around 1300 BC, spindles were invented progressing into production of fabrics and clothes from wool and cotton leading to the establishment of textile industry in 1880s. Plant-based materials namely cotton or wood cellulose fibers were efficiently used by man to create the first synthetic product, Rayon, in 1891 [18].
Notably with the introduction of commercially viable synthetic fiber nylon by DuPont in 1938 by the integration of chemistry and polymeric science, the scope for fiber-based technology saw a great expanse in wide applications [19]. There were many methods developed for producing artificial fibers using polymers under the influence of various physical, chemical, and mechanical processes, where the resultant fibers were found to have limited stretchability and viability. It was Charles V. Boys who in 1887 had reported on the synthesis of fibers using a viscoelastic fluid (beeswax and collodion) under the influence of external electric field [20]. From then on, it was termed electrospinning that facilitated the synthesis of ultrathin fibers having diameters in the nanometer scale. A detailed timeline on the evolution of electrospinning has been schematically represented (Scheme 1).
It was clearly evident from the table that the electrospinning has evolved at a slow pace with the invention of electricity generation and insulation. It was only during the middle of the twentieth century that the development of spinnable polymer solutions and electrostatic work kick started and led to the establishment of markets for artificial fibers. Until 1995, there were considerable activity in patenting aspects and beyond which there was significant progress in the volume of publications and scientific investigations. The publication portfolios generated in two decades from 2000 to 2020 have seen an exorbitant growth in the electrospinning technology and application toward biomedical and tissue engineering sectors (Fig. 1). In accordance, there is a positive trend where the technological inputs have been efficiently transformed into product and processes associated with healthcare settings [104, 145,146,147,148,149,150,151,152,153,154,155,156].
Electrospinning working mechanism
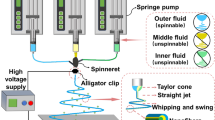
The working mechanism chiefly involves the electrohydrodynamic generation of ultrathin fibers under the influence of high voltage electric supply. In electrospinning process, a liquid drop (polymer/emulsion) is placed under high electric field to generate a jet followed by a sequential stretching and elongation (bending instabilities) resulting in a hyperstretching of the jet called fiber(s) [21, 22]. A schematic representation of the electrospinning process is illustrated in Fig. 2.
Electrospinning process. Formation of Taylor cone by the electrification of liquid droplet which leads to the stretching of the charged jet (flying jet) followed by thinning of the jet into finer diameters owing to bending instability, solidification and collection of fine-tuned jet into fiber(s) onto a rotating metal collector
In the electrospinning process, there are certain key parameters that determine the efficiency of spun fibers, reproducibility, and consistency which include electric field strength, concentration and viscosity of the polymer solution, and spinning distance. The diameter of the fibers and its regulation is expressed by the following equation [23]:
where d represents the diameter of the fiber, \(\gamma\) corresponds to the surface tension, ε represents the dielectric constant, Q codes for the flow rate, I attributes for the current carried by the fiber, and \(\chi\) pertains to the ratio of initial jet length to the diameter of the nozzle.
Although, Fridrikh Model tried to emphasize fiber diameter as a function of only some of the independent parameters, dimensionless index i.e. Berry’s number was considered [24]. It is given as the product of intrinsic viscosity and the concentration of polymer present in the solution. It is this Berry number that had significantly brought in correlation between the electrospinnability and the fiber diameter [25]. The diameter of the fiber can be estimated using the following equation:
where d is the fiber diameter, a represents Mark-Houwink constant, B, the Berry number, and c corresponds to an experimentally determined value related to but not dependent on the crystallinity of the polymer.
Nanofiber generating methods have been categorized based on the spinneret as single-nozzle, co-axial, and multi-nozzle electrospinning as shown in Fig. 3. Single-nozzle electrospinning remains one of the most convenient methods that involves electrification of polymer solution or melt in turn generating fibers mediated through a single orifice (Fig. 3a). This approach facilitates fabrication of composite fibers integrating multiple polymer/solvent system. For instance, sodium alginate-polyethylene oxide (PEO) and chitosan-PEO blends were successfully electrospun for tissue regeneration applications. On the other hand, multi-nozzle electrospinning facilitates assembling of composite nanofibers generated from two or more immiscible polymeric solutions. Here, the nozzles (equipped with two power supplies) are placed either side-by-side or in opposite directions to the grounded collector (Fig. 3c). Yet another modification of multi-nozzle electrospinning is co-axial electrospinning (Fig. 3b) in which the spinneret with one nozzle is placed inside a larger nozzle to achieve generation of core–shell nanofibers, hollow fibers, fibers from non-electrospinnable materials for drug delivery, and protein translocation applications [26,27,28].
a Single nozzle electrospinning involves generation of fibers through electrification of a polymer or melt flow through a single orifice. b Co-axial electrospinning is a modified version of multi-nozzle electrospinning to generate core–shell nanofibers, hollow fibers from non-electrospinnable materials. c Multi-nozzle electrospinning involves assembling of composite nanofibers generated from two or more immiscible polymeric solutions
Scaffold-based electrospinning
The production of electrospun fibrous scaffolds obeys a different hierarchy involving variety of techniques based on the geometric control.
Dual extrusion electrospinning
This dual extrusion electrospinning is a technique used to fabricate multi-layered 3D scaffolds by stacking the microfibrous meshes of the two different feed materials in an alternate fashion to micro/nanomixed meshes. Alongside, this technique enables differential control of two different spatial geometries, namely, the polymeric concentrations and independent solvents. As a result, the micro/nanofibrous structures were made to combine as a single scaffold with definite control over the distribution of electrospun fibers. Hybrid scaffolds of two differently scaled fibers composed of two discrete materials have been fabricated to study the influence on the cellular response of human mesenchymal stem cells seeded on to the scaffold. Further analysis revealed that the electrospun nanofibers could maintain the cellular integrity enabling the deposition of glycosaminoglycan for cartilage regeneration [29]. In a similar fashion, a novel hybrid scaffold was fabricated using electrospinning by arranging the aligned fibers over the random fibers. This well organized scaffold provided uniaxial topographical architecture by the stability and support conferred by the random fibers. These random fibers enable desirable alignment and easy differentiation of cultured C2C12 myoblasts cells [30].
The application of hybrid scaffold for tissue engineering could help surpass the limitation of small pore size, thereby improving the cell migration. For instance, the bone regenerating ability of silk fibroin/poly(ε-caprolactone) nano/microfibrous composite scaffold was found to improve having associated with the nanofiber content in the composite scaffold [31]. Similarly, the cellular response of MC3T3-E1 cells on a micro/nanofibrous mat (PLGA-Col-HA) prepared by dual electrospinning was found significantly higher than on microfibrous PLGA scaffold and micro/nanomixed fibrous PLGA-Col scaffold [32]. Thus, dual extrusion electrospinning technique would be the much sought after approach for fabricating 3D scaffolds with different spatiotopographies and compositions for application toward drug delivery and bone tissue engineering.
Temperature-assisted (cryogenic/melt) electrospinning
Tissue constructs can also be fabricated using temperature driven electrospinning with the help of a temperature controller in order to improve the cell permeability. Cryogenic electrospinning is a technique that uses ice crystals to induce large pores in the electrospun fibers, i.e., it acts as a porogen. This involves the use of low-collecting system to facilitate simultaneous formation of nanofibers and ice crystals resulting in the formation of ice particle implanted fibrous mesh. Then, upon removing the ice particles by freeze-drying, pores are formed inside the electrospun scaffolds. It is by this approach that the porosity and pore sizes are varied by the size and amount of implanted ice crystals. The higher the amount of implanted ice crystals, the greater was the increase in porosity. One of the most important parameters, the humidity, was found to regulate the state of the ice crystals in the electrospinning environment. The scaffold pore size can be adjusted in the range of 10 to 500 μm which enabled the cellular infiltration of NIH 3T3 fibroblasts into a 50-μm-thick porous scaffold under static culture condition within 7 days and proliferated at large within a period of 14 days [33]. This crystal induced scaffold when placed into rat dorsum showed significant improvement in the infiltration of macrophages and collagen-producing fibroblasts in 56 days.
Furthermore, cryogenic electrospinning was recruited in the chemoresistance of cancer cells. For instance, cryogenic electrospun silk fibroin constructs were fabricated to mimic the cancerous extracellular matrix (ECM) onto which HN12 (human head and neck squamous cell carcinoma) cells were seeded to investigate the cell-to-matrix interactions and drug resistance. The highly porous nature of the cryogenic electrospun scaffold significantly supported cellular infiltration in a well-protected fashion. This approach mimicked the 3D culture model facilitating the replication of cells, differentiation, and infiltration interspersing the scaffold [34].
Cryogenic electrospinning mechanism works by blowing the polymer solution into cryogenic solution using pressurized gas channelized through concentric nozzles. Adopting this approach, fibrous scaffolds with controlled porous structures were fabricated by combining thermally induced phase separation and solution blow spinning. These fibrous scaffolds are as a result of the ice microspheres that lead to the formation of interconnected networks with the fibers assembled directly onto the liquid nitrogen surface. Alongside, they tend to exhibit 3D architecture with interspersed macroscale pores [35]. Recently, porous fibers with extra nanotopography (with diameter 3.29 ± 0.42 μm) have been fabricated using self-made cryogenic electrospinning system. The pores embedded were found to exhibit pits and polygon concaves on their surface. The formation of pits and polygon concaves were induced by phase separation at freezing temperature and solvent interaction with ice crystals [36].
Melt electrospinning on the other hand constitutes another temperature propelled electrospinning technique utilizing higher temperature. This technique harbors a polymer melt in place of polymer solution to enable controlled fibrous deposition of 3D scaffolds with utmost precision in porosity and alignment. In accordance, the polymer placed in a syringe would be subjected to heating at ~ 400 °C and blown using air pressure. This technique is more advantageous as the use of most toxic solvents is circumvented. For instance, melt electrospinning was employed to deposit PCL over structured metallic collector substrates to produce batch-to-batch scaffolds with a mean fiber diameter of 15 μm and a pore size of ~ 250–300 μm on the concave side and ~ 20–80 μm on the convex side. When osteoblasts were seeded onto the PCL scaffolds, there was significant cellular infiltration, growth, and differentiation that mimicked the 3D environment [37].
Recently, Zhao et al. [38] have reported on a self-powered hand-held melt electrospinning device for in situ e-spinning on wounds directly. It is by the use of special high heat transfer insulation unit that the problem of electrostatic interference was surpassed. Moreover, the device is simple to use, reliable, and safe in handling due to its small volume of 24 × 6 × 13 cm3 weighing about 450 g. Most importantly, biocompatible and biodegradable polymers (polycaprolactone) were successfully e-spun into fibers directly on wounds serving as a dressing gun. Moreover, Großhaus et al. [39] have fabricated medical-grade poly(ε-caprolactone) adopting melt electrospinning by modifying the nozzle. This modification had led to the fabrication of fibers with the smallest mean diameter of ~ 275 ± 86 nm under optimized conditions. This was achieved by positioning a small acupuncture needle to reduce the flow rate up to ~ 0.1 μL h−1 and by making the sharp tip protrude beyond the nozzle into the Taylor cone. To retain the material quality, the device was coupled with a dual head printer, and then the melt electrowriting could be performed to produce smallest melt electrospun fibers.
In recent years, melt electrospinning writing (MEW) is gaining popularity as this technique involves solvent-free fabrication of polymeric scaffolds. Moreover, the scaffolds with a large surface area, high porosity, and controlled deposition of the fibers could be fabricated using this technique. This was demonstrated by fabricating cell-scaffold constructs using poly(ε-caprolactone) using MEW for seeding primary human-derived dermal fibroblasts [40].
Needle-less electrospinning
The needle-less electrospinning system has been developed to address the limitations encountered in needle-based electrospinning system. This system is found capable of enhancing the production capacity of fibers by motivating numerous jets simultaneously from the free surface of the liquid or protuberances with the aid of high voltage that typically acts as fiber generators. For instance, Lukas et al. [41] have reported on the self-assembly of charged jets propelled out from the free surface of the liquid. The critical field strength Ec was given by \({E}_{c}= \sqrt[4]{4\gamma \rho g}/{{(\varepsilon }^{'})}^{2}\) where γ represents surface tension, ρ corresponds to gravity acceleration, ε’ signifies permittivity, and g implies acceleration due to gravity. It has been reported that the more jets produced, the lesser became the distance between individual jets when electric field was made stronger for the given solution. Moreover, the needleless spinning involves strict participation of spinneret and jet initiation from the liquid surface. But the criticality relies on making the electrical forces focus toward the surface of the solution prior to needleless spinning [42]. The parameters involved in needle-less electrospinning on a par with needle-based electrospinning have been enlisted in Table 1.
Ultrasound-enhanced electrospinning (USES)
One of the novel electrospinning techniques employed for the fabrication of nanofibers is ultrasound mediated electrospinning. This was the most recent technology which was patented by Laidmäe et al. in 2016 [165]. It is a continuous orifice-less technique that recruits high-intensity focused ultrasound to enable nanofiber synthesis from a free polymeric solution. The conventional electrospinning technique showcased some limitations such as needle clogging and precision control of the fiber properties which could be overcome by this novel approach. Ultrasound bursts were capable of generating a liquid protrusion with a Taylor cone from the surface of the polymeric solution (e.g., polyethylene oxide). When the polymer has been imparted with a high negative voltage, these nanofibers could jet off from the protrusion tip and successfully land on to the electrically grounded collector. The ease of controlling the ultrasound characteristics facilitated physical modification of the fabricated nanofiber at its topographical level in a non-chemical fashion. There was some phenomena viz. formation of ultrasound fountain relative to applied electric field, generation of capillary waves by ultrasound, cavitation, acoustic streaming, and thermal effects [43]. Similarly, Hakkarainen et al. [44] have investigated fabrication of nanofibers using USES and compared with the one synthesized using the traditional electrospinning method for drug delivery applications. The nanofiber generated using USES had higher fiber diameter of 402 ± 127 nm over 77 ± 21 nm fiber synthesized using traditional method. Moreover, the increase in burst count had significantly increased the diameter of the fiber generated using USES up to 555 ± 265 nm with variation in the fiber size. The fibers generated using USES showed promising alternative over aqueous-based fabrication for drug delivery applications. Moreover, this approach confers numerous advantages such as generation of beadless fibers, ease of controlling the fiber diameter by regulating the cycles per ultrasound pulse, and fabrication of amorphous fibers which could not be achieved in traditional electrospinning.
Feed materials for electrospinning
Electrospinning involves the utilization of mostly organic polymers in the form of a solution or a melt to generate nanofibers. Besides, small molecules are also efficiently electrospun into fibers, provided that they exhibit the property of self-assembly and chain entanglement. Moreover, the involvement of sol–gel chemistry has taken electrospinning to a different level in generating fibers from composite materials. Notably, materials constituting different dimensions/morphologies such as nanoparticles, nanowires, and nanotubes can also be directly electrospun into fibers.
Polymers
Organic polymers are the most facile materials that can be directly used in electrospinning process. There are certain characteristics such as solubility, viscosity, electrical conductivity, spinnability, stability, reconcilability, dissolution into appropriate solvent system, and the recruitment of high molecular weight polymers that determine the success of electrospinning.
There are two methods, namely, solution and melt electrospinning, that utilize organic polymers as feed. Solution electrospinning is the most common method, where a jet of organic polymer solution is stretched, elongated and thinned by innumerous bending (whipping) instabilities. This is followed by the evaporation of the solvent, solidification of the jet, and deposition of fibers in the form of non-woven mat onto the grounded collector [45]. On the other hand, melt electrospinning involves direct generation of nanofibers from the melts of the solvent insoluble polymers such as polyethylene and polypropylene [46]. Although electrospinning process generates nanofibers with thinner diameter and varied architecture, the choice of the feed materials plays a vital role in deciding the functional aspects for biomedical applications. A list of commonly used polymers and their appropriate solvents used in electrospinning has been shown in Table 2.
Natural polymers
The natural polymers are derived from either plant or animal bodies and are considered excellent renewable resources possessing biodegradability and biocompatibility properties for biomedical and tissue engineering applications. Natural biopolymers such as DNA, silk fibroin, chitosan, chitin, hyaluronic acid, collagen, alginate, and dextran have been successfully electrospun into nanofibers. Besides plant and animal based polymers, the ones derived from sea weeds were also employed; in particular, the alginates with cross links are used as a potential biomaterial in fabricating scaffolds and drug cargoes [47]. In this line, an excellent biomaterial chitosan is obtained by the deacetylation of chitin where the extent of deacetylation and pH define its charge density. Notably, the positively charged polymer has increased affinity toward negatively charged drugs or proteins that facilitates its wide applications toward drug delivery [48].
Beyond the incredible features of chitosan based polymeric scaffolds, they still lack sufficient mechanical properties for application toward tissue engineering applications. But, by structural configuration, ease of derivatization, and combination with other polymers, their mechanical properties could be restored. For instance, the surface modification of chitosan with hyaluronic acid had shown significant improvement in mechanical, biological, and non-degrading properties. These properties have extended the application of chitosan-based scaffolds in would dressings, skin grafting, tissue engineering, and drug delivery applications. In a similar fashion, nanofibrous membranes were fabricated by electrospinning chitosan and poly(vinyl alcohol) with antibiotics loaded at different ratios. The volumetric ratio of chitosan-PVA on the nanofibrous structure was found to be 50/50 as revealed by SEM. This composite electrospun scaffold had great potential as wound dressing to prevent infection in skin tissue regenerative procedures [49].
A bicomponent nanofibrous scaffold was fabricated by photocrosslinking of maleilated chitosan-methacrylated poly(vinyl alcohol) via electrospinning with improved water stability. The water stability test revealed that the electrospun photocrosslinked matrix formed in the ratio of 10:90 had excellent integrity of the fibrous structure. Alongside, the cytotoxic studies performed using L929 cells showed that the nanofibrous scaffolds exhibited excellent cytocompatibility for potential wound dressing application [50].
On the other hand, electrospun mats containing PVA-chitosan and PVA-chitosan-tetracycline hydrochloride (TCH) were fabricated by Alavarse et al. [51]. The electrospun fibers had shown even distribution of the drug along the fibers with significant thermal and morphological characteristics. These fibers facilitated burst delivery of the drug during the first 2 h typically exerting antibacterial activity toward Escherichia coli and Staphylococcus aureus. In vitro studies using rabbit aortic smooth muscle cells (SMCs) were carried out to investigate the cell viability and adherence of the cells over the scaffold by performing indirect contact MTT assay and scratch assay. The results revealed excellent biocompatibility with potential antibacterial action to hasten wound healing serving as a wound dressing.
Chitosan-based nanofibers have also been fabricated for diabetic wound healing applications. In this line, Ahmed et al. [52] have prepared nanofiber mats by electrospinning chitosan-polyvinyl alcohol (PVA)-zinc oxide solutions. These nanofibrous mat had a mean diameter size of 891.72 ± 10.65 nm at the cross over points and 279.34 ± 7.23 nm at the non-crosslinking points but remained finer and smoother. Moreover, the nanofibrous mat exhibited potential antibacterial activity against E. coli, P. aeruginosa, B. subtilis, and S. aureus. The wound healing activity of the nanofibrous mat in diabetic animal model showed significant wound closure of 90.5 ± 1.7% in day 12 showcasing as a promising dressing material for diabetic wounds. In a similar fashion, a wound dressing was fabricated by electrospinning PVA-chitosan-starch into nanofibrous mats and the healing property investigated. The nanofibrous mats exhibited high porosity of 91%, which was further controlled by the addition of starch. The mean surface roughness of the nanofibrous mats were found to be in the range of 262–435 nm. The fabricated nanofibrous mats showed in vitro degradation when immersed in PBS for 21 days characterized by thinning of the fibers pertaining to effective crosslinking of the mats. Moreover, there was a significant antibacterial effect in the range of 60–84% and 47–72% respectively for Gram-positive and Gram-negative strains respectively. The scratch assay demonstrated efficient migration of L929 cells from side to side of the artificial wound within 24 h [53].
Hyaluronic acid is one of the emerging and promising candidates investigated for tissue regeneration application. Hyaluronan is a linear anionic polysaccharide largely found in human tissues in the form of non-sulfated glycosaminoglycans (GAG), mainly involved in the regulation of cell adhesion, proliferation, and differentiation. It is one of the chief components of the extracellular matrix (ECM) having larger interactions with the key proteins present in the ECM. They are widely investigated in the fabrication of scaffolds in tissue engineering. Alongside, due to the polyelectrolytic nature, the solution turns more viscous making electrospinning very difficult. To achieve critical chain entanglement, HA fibers were introduced into sodium hydroxide-dimethylformamide system to generate fibers of 100 nm [54, 55].
In addition, the high surface tension of HA prevents the formation of highly concentrated solutions making the evaporation of water very difficult. In order to resolve, there were numerous attempts made to electrospin HA into nanofibers. One of the common approaches to electrospin polymers with low spinnability is by the use of a dragging polymer, with high spinning characteristics. This approach provides a core–shell nanofiber when HA solution was blended with chitosan [56]. Moreover, the addition of surfactants or a different solvent could help overcome the problem of high surface tension as demonstrated by Malkin et al. [57]. In their study, they concluded that the electrospinning stability could be achieved only below the critical concentration prior to the use of desired intermediate solvent. As a result, there was a positive effect on the spinnability by polymer–solvent demixing solidification mechanism.
Vitková et al. [58] have fabricated nanofiber electrospun using HA combined with PVA and PEO by using two intermediate solvent mixtures (water and isopropanol; water, ethanol, and methanol). Both these solvent mixtures facilitated electrospinning of HA of lower (600 kDa) and higher (1180 kDa) molecular weights, among which the lower molecular weight HA showed higher tendency to form spherical shaped particles. But the best results were acquired when HA at higher molecular weight was used and which was characterized by smooth fibers of a diameter of 100 nm making them promising candidates for tissue engineering applications.
Recently, Fuenteslópez and Ye [59] have fabricated electrospun fibers of HA-chitosan using a portable device. Their work typically demonstrated the electrospinning of HA and chitosan nanoparticles together with polymers such as PCL and gelatin with the aid of a portable device, the Oxford Portable Electrospinner (OPE). The polymeric blends were electrospun at random or aligned fiber arrangements by the device facilitating in situ fiber deposition on the targeted site. The cell viability experiments conducted using these electrospun nanofibers showed cytocompatibility up to 72 h. Moreover, the unidirectional arrangement of fibers helped the cells in guiding the proliferation of cells in a uniaxial fashion corresponding to nerve or muscle tissue repair. Altogether, the HA-chitosan blended polymeric nanofibers exhibited excellent biocompatibility, biodegradability, and non-immunogenicity making them promising carriers for drug delivery and tissue repair applications.
Collagen and its derivatives such as gelatin and other polypeptides are considered natural polymers predominantly found in the connective tissues of humans, spider silk, and mori silk. They are investigated for their multifunctional polymeric properties. Converting collagen into fibers offer good mechanical properties, porosity, and biocompatibility. They have been largely employed in tissue regeneration applications such as artificial skin graft, vasculature, tissue (cartilage) repair, periodontal restoration, and wound dressings [60].
One of the major advantages in fabricating electrospun collagen-based scaffolds is that they readily mimic the microscale architecture of native extracellular matrix present in the dermis. Collagen-based scaffolds are often utilized in wound regeneration and tissue engineering applications. For instance, collagen and chitosan have been electrospun into nanofibers and been applied to enhance angiogenesis and epithelialization of scalds in rat model [61]. In a similar fashion, Türker et al. [62] have fabricated electrospun hybrid scaffold comprising of collagen and poly(l-lactide-co-ε-caprolactone) (PLLCL) for 3D cell culture applications. Co-spinning approach was adopted to fabricate biomimetic scaffold by simultaneously electrospinning collagen and PLLCL, facilitated by a scarifying agent, polyvinylpyrrolidone (PVP). This electrospun hybrid scaffold exhibited 3D network structure with a 300–450-nm diameter for maximized cell adhesion of NIH 3T3 mouse fibroblast cells. Such biomimetic architecture showed a major impact of cell proliferation and viability on a par with 2D systems.
Li et al. [63] have fabricated radially aligned electrospun collagen-poly(ε-caprolactone) mats to accommodate gradients of stromal-cell-derived factor-1α (SDF-1α). These gradients were produced continuously in a controlled and reproducible fashion by regulating the collector size and time during the process of electrospinning. Fabricating a long-term gradient was facilitated by using SDF-1α with unique peptide of collagen-binding domain (CBD), capable of specific binding toward collagen. The results revealed that CBD-SDF-1α gradient scaffolds guide the endogenous neural stem cells to migrate from the periphery to the center along the lined up electrospun fibers. Such collagen-based nanofibrous scaffolds find its potential application toward nerve regeneration.
Guo et al. [64] have studied the physiochemical and biocompatibility properties of electrospun collagen-chitosan membranes applied toward guided bone regeneration. Apart from physiochemical characteristics, in vivo calvarial bone defect created on rats and the regenerative efficiency were investigated. The electrospun collagen-chitosan membranes showed higher tensile strength and more stable degradation rate. During the fourth and eighth week, ELISA was performed to quantify the bone alkaline phosphatase and osteocalcin. The animal model on which the electrospun collagen-chitosan membranes were applied showed higher levels of alkaline phosphatase and osteocalcin in the fourth and eighth week respectively. Alongside, the radiographical and histological results revealed osteogenesis (new bone formation) with characteristic higher bone volume, trabecular number, and lower trabecular spacing.
Synthetic polymers
There are over 100 different types of synthetic polymers used for the direct generation of nanofibers adopting electrospinning. Some of them have been commercially used; in particular, polystyrene and poly(vinyl chloride) were used for environmental applications. Alnaqbi et al. [65] have generated nanofibrous sorbents adopting polymer blending strategy for the removal of various oil spills.
Indeed biocompatible, biodegradable, non-toxic, and mechanically stable synthetic polymers are largely been employed for the generation of nanofibers using electrospinning and used for biomedical applications. Few of them include poly lactic acid (PLA) and PLGA which can be directly spun into fibers and used as scaffolds in tissue engineering [66]. PLA offers the advantage of dissolving easily in different kinds of conventionally used solvents such as acetone, chloroform, dichloromethane, dimethylformamide, and tetrahydrofuran. It is prepared using condensation and ring opening polymerization techniques. As the former method would produce polymer with low molecular weight and poor mechanical properties, the latter would be the most preferred technique that introduces excellent mechanical stability [67, 68]. Alongside, in the derivatives of PLA, namely, poly-L-lactic acid (PLLA) and PLGA, the process occurs via co-polymerization with L-lactide and polyglycolic acid respectively [69, 70]. These polymers when converted into nanofibers have profound implications in biosensors and molecular filtrations and been used even in preserving biological specimens [71, 72].
Conducting polymers constitute one of the recently emerged classes of polymers possessing remarkable properties on a par with the conventional polymers. Their characteristic π-conjugated backbone confers enough electrical conductivity upon charge transition by redox reactions. They possess typical characteristics of both the metallic (electrical and optical properties) and polymeric materials (biocompatibility, good processability, chemical stability) [73]. Polyacetylene was the first recognized conductive polymer in the 1970s and has been widely explored for its biophysical properties [74]. Due to the unstable nature and difficulty in processing of this polyene, some of the alternative forms have been introduced such as polyaniline, polypyrrole, polythiophene, and poly(3,4-ethyelenedioxythiophene) with improved thermal stability and conductivity [75]. Moreover, the biodegradable property has been introduced into conducting polymers by the copolymerization of aniline. Notably during electrospinning, these conducting polymers are mixed with biodegradable polymers and electrospun into nanofibers in the fabrication of scaffolds.
Besides innumerous conducting polymers, there are only few polymers that have been successfully developed into nanofibers. For instance, polypyrrole was subjected to pre-processing to attain adequate molecular weight by dissolving it in dimethylformamide and by the addition of di(2-ethylhexyl). Upon electrospinning, the polypyrrole was developed into nanofibers endowed with a thinner diameter of 70 nm [76]. Secondly, direct electrospinning of PANI with PLA/gelatin was developed into nanofibers imparting an electrical conductivity of 4.2 × 10–3 S cm−1 and has been used for cardiac tissue engineering applications [77, 78]. There are different functional polymers such as polyvinylidene fluoride and polyvinylidene fluoride-co-trifluoroethylene with enhanced piezoelectric and pyroelectric properties that can be directly electrospun for energy harvesting and biosensor applications [79, 80].
Reactive polymers
Natural and synthetic polymers have been widely used in electrospinning for the development of fibers. In recent years, focus towards the synthesis of reactive and functionalizable nanofibers has drawn much attention. For instance, conjugating appropriate small molecules, ligands, and/or biomolecules into the nanofibers have introduced functional aspects in the generated fibers (Fig. 4). Recent advancement in controlled polymerization techniques has facilitated the fabrication of nanofibers with functional aspects. Moreover, nanofibers developed from polymers with reactive functional groups can undergo post-polymerization modification even under mild conditions. Such fibers offer excellent platform for functionalization of wide variety of polymers intended for desired biomedical applications. The functionalization of the polymers are achieved either by adopting covalent and non-covalent methods. Encapsulation and chemical-mediated attachment facilitate incorporation of active agents (drugs), biological moieties (enzymes, proteins, extracellular matrix etc.), and growth factors for varied applications.
Moreover, fabrication of functionalizable nanofibers is achieved by post-spinning activation of polymers or by direct spinning of reactive and clickable polymers. Conventionally, the surface modification is done by surface activation processes such as plasma treatment or wet chemical methods. This approach poses challenges in achieving homogenous surface activation in turn compromising the fibers stability. To surpass these challenges during post-spinning fiber modifications, recruitment of polymeric precursors possessing specific reactive-functional groups would be the most appropriate strategy in introducing the functional aspects directly into the electrospun fibers. This was facilitated by click chemistry that led to the synthesis of wide variety of reactive polymers. Some of the most commonly used post-polymerization modification reactions include the following: Huisgen-type copper-catalyzed azide-alkyne cycloaddition (CuAAC), Michael-type nucleophilic thiol-ene conjugate additions, the Diels–Alder, inverse electron demand Diels–Alder cycloaddition, strain-promoted azide-alkyne cycloaddition (SPAAC), and thiol-ene conjugate additions [81,82,83].
There are instance of fabrication of covalently crosslinked and amine-reactive microcapsules in the form of layer-by-layer assembly of reactive polymers. This has been accomplished by alternating the adsorption of interacting polymers on the surface. Fabrication of such assemblies finds vast application toward catalysis, nanofiltration, preparation of hydrophobic and antimicrobial surface coatings, biomedical device coatings, and drug and gene delivery. This approach was demonstrated by encapsulating high molecular weight FITC-dextran in BPEI/PVDMA (branched poly(ethylene imine)/poly(2-vinyl-4,4-dimethylazlactone)) capsules [84]. Similarly, Broderick et al. [85] have fabricated layer-by-layer assembly of oligonucleotide and protein arrays onto multi-layered reactive polymers comprising PEI and amine reactive, azlactone-functionalized PVDMA. This assembly could efficiently hybridize complementary sequences with high signal intensities and with high sequence specificities. The azlactone groups present in the set up was exploited to immobilize proteins and to fabricate functionalized arrays of proteins and enzymes. Such type of approach has enabled the development of new assay format viz. application toward biomolecular arrays.
Materials fabricated with fascinating properties are most sought in biomedical, environmental, and industrial applications. As with growing incidences of bacterial colonization, infections, and resistance to conventional antibiotics, a novel approach of non-biocidal means has gained significance. Bacterial quorum sensing remains one such target connected with virulence, which need to be countered in order to limit growth and reduce infections. A nanoporous, polymer-based superhydrophobic coatings was fabricated to encapsulate a potent, water-labile peptide-based quorum sensing inhibitors in Staphylococcus aureus. The peptide-based quorum sensing inhibitors were released in a controlled fashion over a period of 240 days, and they could strongly inhibit agr-based quorum sensing in S. aureus. Fabrication of such materials through electrospinning process has enabled non-bactericidal approaches for the long-term attenuation of quorum sensing–mediated bacterial phenotypes [86].
In a similar fashion, non-woven polymeric nanofibers have been encapsulated with quorum sensing inhibitor to inhibit quorum sensing and virulence in S. aureus. This quorum sensing inhibitor (a macrocyclic peptide) has been loaded onto a degradable polymeric nanofibers using electrospinning. As a result, the inhibitor when kept under physiological conditions was found to be released over a period of 21 days, thereby exhibiting agr-based quorum sensing inhibition in S. aureus at least for 14 days and without inducing cell death. Furthermore, these materials were also found to inhibit production of hemolysins, quorum sensing–controlled virulence phenotype, and reduced lysis of erythrocytes. Such quorum sensing inhibitor–based strategy has led to the development of novel anti-infective materials and therapeutic strategies targeting virulence [87].
Small molecules
They are one of the challenging materials which can be directly made into nanofibers using electrospinning. Their unique self-assembling characteristics with a significant chain entanglement capable of stabilizing the electrified jet and suppression of Rayleigh instability could help achieve typical nanofiber. Notably, the small molecules’ structure-concentration interrelationship and the type of solvent decide the success of electrospinning for the generation of fibers. Mostly, the small molecules involved in the development of nanofibers constitute amphiphiles and cyclodextrin derivatives [88]. Most importantly, lecithin, a zwitterionic mixture comprising glycerophospholipids and phosphatidic acid, was the first reported small molecule to be developed into nanofibers. Its hydrophobicity and surface tension reduction potential have made them the suitable candidates for regenerative applications, thereby modifying the structure and surface area of the scaffolds [89].
Secondly, their self-assembling properties tend to form spherical micelles above the critical micelle concentration (CMC). By increasing the concentration further, the morphology undergoes a transition from spherical to columnar structures which again overlaps to form chains resembling fibers. The criticality relies on the concentration of lecithin to the amount of solvent used in achieving the desirable fiber diameter. For instance, the ratio of lecithin:CHCl3:DMF at 70:30:43 (wt%) yielded continuous fiber with an average microscale diameter of 2.8 μm whereas the concentration of DMF when increased to 50% (> CMC), the fibers generated had a diameter of 5.9 μm [90]. In addition, Gemini ammonium surfactants N,N′-didodecyl-N,N,N′,N′-tetramethyl-N,N′-ethanediyldiammonium dibromide (12–2-12) in H2O-CH3OH have been successfully electrospun into micellar microstructured hydrophilic nanofibers with diameters in the range of 0.9 to 7 μm [91, 92].
Recently, peptide derivatives of the pyrazole-isothiazole scaffold have been specifically fabricated using electrospinning. For instance, phospholipid amphiphiles, tetraphenylporphyrin compounds, and cyclodextrin small molecular system have been successfully electrospun into nanofibers [93]. As mentioned earlier, the type of the CD used and its concentration decide the morphology and the fiber diameter. The disadvantage of the formation of bead-like nanofibers could be overcome by using CD owing to its tendency to form aggregates via hydrogen bonding and exhibiting high solution viscosity and viscoelastic solid-like characteristics [94]. Moreover, the CD offers truncated cone-shaped and relatively hydrophobic cavity in which the drug of choice can form inclusion complex with hydrophobic drugs with hydrophilic exterior, thereby increasing the water-solubility of drugs for prolonged delivery applications [95]. Xiang et al. [96] have developed hydroxypropyl-β-cyclodextrin-polyvinylpyrrolidone-loaded resveratrol nanofibers to enhance the water solubility of resveratrol and to remain stable under UV irradiation. In vitro studies conducted have revealed its slow and sustained release via nanofiber extrusion. The ratio of HP-β-CD to PVP at 1:2 yielded nanofibers with smooth surface and uniform thickness. Further, upon optimizing the concentration of resveratrol to 5%, the solution viscosity corresponded to 4.06 Pa.s attributing for good fiber morphology with an average diameter of 500 nm.
Colloids
Colloidal particles comprises of homogenous non-crystalline substance either found in dispersed or continuous phase. They may include gels, sols, and emulsions where they can be successfully electrospun into nanofibers, once they are capable enough to form entanglement among the dispersed particles to form a jet. This colloid-electrospinning has been a widely used technique to immobilize the particles in fibers at nanoscale dimension. The essential criteria for electrospinning rely on the size and viscosity facilitated by hydrolysis or condensation process. Most importantly, the viscosity tends to be one of the important parameters deciding the size/thickness of the fibers generated. Conversely, sol–gel method was the typical alternative and widely used method for the generation of nanofibers. For instance, by using tetraethyl orthosilicate (TEOS), distilled water, ethanol, and HCl at the molar ratio of 1:2:2:0.01, the silica sol was successfully electrospun into fibers employing acidic catalysis reaction [97]. The thickness of the fibers generated was found to be in the size range of 200–600 nm with an applied voltage of 12–16 kV. Similarly, lithium-cobalt acetate nanofibers with diameter ranging from 0.5 to 2 μm were generated by calcination method [98]. This method was employed to synthesize metal oxide nanofibers comprising aluminum, zinc, nickel, and cobalt at micrometer scale dimension owing to limited control over the size and uniformity of fibers influenced by the rheological properties of the sol [99].
Further, metal nanoparticle comprising silver known for its antimicrobial properties have been electrospun by integrating with synthetic polymers such as PEO, PVA, PVP, and polyacrylonitrile [100] or with natural polymers such as chitosan, gelatin, and N-carboxyethylchitosan [101]. For instance, in situ reduction of silver nanoparticles using formic acid as a solvent was integrated with polyamide 6 and electrospun. This nanoparticle incorporation has attributed for enhanced resonance spectroscopy (SRS) [102]. It was Chen et al. [103] who unraveled the mechanism behind the nanoparticle-polymer interaction where the π-π co-ordination between the silver moiety and polymer facilitates photoexcitations and charge transfer endorsing optical application.
Applications of electrospinning in biomedical and environmental sector
Prior to thorough understanding on the fundamentals related to physical and functional properties of electrospinning materials, this section attempts to bring out the implications of nanofiber technology toward biomedical and environmental sectors. Nanofibers are mostly applied in fabricating scaffolds for either tissue regeneration or effective drug delivery. Conventionally, tissue regeneration was made possible by involving auto- and allografts. Autograft, although genetically feasible, pose greater challenge toward the availability of donor sites in case of larger affected area. As this could not solve the purpose of grafting and inturn the donor may suffer heavy damage due to the removal of tissues. Besides, the possibility of rejection would be greater when a genetically different material is introduced in case of an allograft due to immunological response. To counterbalance the regeneration and compatibility, this novel tissue regeneration approach has established a biocompatible arena for the host tissues to adhere, proliferate, and differentiate into specific tissues that need to be repaired [105]. This could be achieved by the nanofibers for its enhanced surface area and porosity that drives the regeneration efficacy of the scaffolds [106].
Tissue engineering
The requirements for successful growth of tissues via nanofiber scaffolds produced using electrospinning have made this technology the most preferred one. Properties such as biocompatibility, biodegradability, large surface area, maintaining the structural integrity, high porosity, and high mechanical stability have improved the growth and differentiation of cells [107]. Mostly nanocomposite materials similar to extracellular matrix (ECM) proteins (collagen and glycosaminoglycans) are preferred for their improved cell function and cell–cell/cell-ECM affinity. Notably, thinner fibers with diameter in the range of 50–200 nm have been reported to enhance cell adhesion, proliferation, and alkaline phosphatase activity [108]. Furthermore, the unique properties conferred by these core–shell nanofibers and their ease of manipulation pertaining to mechanical and electrical properties have augmented the scope for tissue engineering. The incorporation of antimicrobial nanoparticles such as Ag, Au, Zn, Ti, Mg, Cu, SWCNT, and graphene [109,110,111] and polymers (chitosan) [141] into the nanofibers via electrospinning have profound implications in the fabrication of biomedical devices, textiles, and tissue engineering. Some of the nanofiber scaffolds that have been successfully electrospun and extended to tissue engineering applications have been enlisted in Table 3.
In tissue engineering applications, there is a novel in situ approach that holds promise in the regeneration of functional blood vessels following the guidance of vascular scaffolds. The tubular design with multi-layered wall mimicking the native blood vessel architecture has been the most sought after model [112]. It is in this model that the tunica intima takes care of the functional properties by accelerating endothelialization and preventing thrombus. Secondly, the media confers mechanical stability to the vascular scaffold at the anastomotic sites and thereby avoids architecture remodeling. In order to achieve better endothelialization, critical evaluation on the inner and middle layers of the tubular shape needs much consideration. This greatly helps in supporting increased blood flow without leakage in the lumen and checks for anticoagulation properties in preventing stenosis and occlusion that determine the lumen patency [113].
For instance, nanofibers with introduction/loading of heparin or arginine-glycine-aspartic acid have been used to fabricate lumen to evade early thrombosis [114]. Recently, Eilenberg et al. [115] have designed a novel degradable thermoplastic polycarbonate urethane (dPCU) grafts for small vessel replacement in rodents. They have reported an upregulated anti-inflammatory signaling in dPCU conduits offering excellent patency rates of 92.9% without causing any adverse effects in rat model. When compared to expanded polytetrafluoroethylene (ePTFE), dPCU grafts accelerated transmural ingrowth of vascular cells into a structured neovessel around the graft with gradual reduction of graft material. In addition, natural biopolymer silk has shown promise in the fabrication of vascular scaffolds using a bi-layered Antheraea assama (AA) and Bombyx mori (BM) silk. This hybrid model with its inner layer measuring 40 μm and interconnected pores has accelerated cellular infiltration whereas the outer dense layer offers mechanical stability.
Human adipose tissue-derived stromal vascular fraction seeded silk vascular graft was implanted surgically in Lewis rats as an abdominal aortic interposition graft. It was inferred that AA silk laden vascular graft showed superior animal survival and graft patency after 8 weeks. Further, these silk laden vascular grafts degrade into amino acids, and their resorbable byproducts well elucidates its remodeling ability [116]. This approach remains to be the futuristic vascular alternative for bypass and reconstructive surgeries.
The functional properties of the electrospun nanofiber-based scaffold have been improved with respect to architecture and mechanical properties with the advent of 3D electrospinning. Such manipulations find their application toward cartilage, bone, tendon, ligament, skeletal muscle, nerve and cardiac tissue regeneration (Table 4). Fabrication of 3D structures confers cell-permeable structure with controlled thickness mimicking biological niches to guide cell growth, differentiation, and tissue regeneration. Characteristic features such as mechanical strength, suture retention strength, and degradability have at large been employed for heavy duty tissues such as muscle, tendon, and ligament.
Orthogonally oriented scaffolds have gained popularity and criticality in engineering of intestinal smooth muscles. Moreover, the directional alignment of the cells either on x–y, x–z, and y–z plane with desired structure and functions have been the most sought after approach. [117] have examined the cellular arrangement on different planes of scaffolds made of two layers of orthogonally oriented fibers. This was one of its kinds where the cells were manipulated to align inside the 3D scaffolds in vivo using a two-layer ePCL scaffold with orthogonally aligned fibers mimicking the intestinal circular and longitudinal muscle layer. Moreover, there was an enhanced regeneration and alignment of the muscle layers not only on the surface but also inside of the ePCL scaffolds viz. at x–z and y–z planes.
Similarly, Wang et al. [118] have formulated a serum-free culture methodology to maintain the gut motility and intestinal regeneration with peristaltic function. This approach was adopted to maintain the spontaneous and periodic contractions of murine and human intestinal muscularis cells. A 11% (w/w) poly-caprolactone was subjected to electrospinning and coated with neutralized collagen. The expression of mature marker enabled organized arrangement of the cell sheets facilitating the co-existence of mucosa, muscularis, and serosa. Moreover, the epithelial cells were stretched by the contracting muscularis cells pertaining to gut motility disorders and functional regeneration of the engineered intestinal cells.
Wound healing
An injury hindering the vital functions of the tissues is termed as a wound. Skin remains as the front-line defense of human, and the largest organ is the most susceptible part responding toward physical means of injury. The severity of the wound determines the type of approach adopted to hasten healing process. For instance, minor wounds would heal faster through intrinsic repair mechanism, whereas large-scale or full-thickness wounds (burns, diabetes-related wounds) require scaffolds to aid migration, proliferation, and maturation of repairable cells [119, 120].
Besides wound healing, anti-inflammation, anti-infection, scar formation, and conditions leading to skin cancer need timely resolution. This has been facilitated by the nanofibers generated using electrospinning for faster healing of wounds. These nanofibers with its typical topography mimicking basket weave-like pattern of collagen have been fabricated to accelerate migration and infiltration of repairable cells. Most importantly, the orientation of the fibers play a key role in expediting the process of wound healing. Intercross nanofibers on a par with random or uniaxially aligned fibers exhibit best healing performance by accelerating the infiltration of fibroblasts and keratinocytes [121]. Pal et al. [122] have demonstrated the wound healing efficiency (3 weeks) in rat model by fabricating chitosan-PCL core-sheath fibers using emulsion electrospinning.
Further, the use of 3D scaffold in the regeneration of dermal ECM was found critical. To overcome infiltration intricacies, sandwich-type scaffold was preferred over 3D in skin regeneration application. Here, the radially aligned nanofibers are placed at the bottom, a mat comprising an array of square shaped microwells at the top, and the microlevel skin tissues placed in between the two layers. Their applicability as a promising wound dressing facilitates enhanced cell infiltration, thereby preventing drainage at wound site [123]. Besides 2D nanofibers, fabrication of 3D nanofiber scaffold to promote cellular infiltration with controlled thickness and porosity has been demonstrated by Jiang et al. [124] using depressurized subcritical CO2 fluid. Such modified 3D scaffold not only formed layered structures but also retained the fluorescent intensity and antibacterial efficacy of coumarin 6 and LL-37 peptide. This helped to surpass nanotopographical cues and significantly accelerated cell infiltration and neotissue formation aided by subcutaneous implantation. Promising results in the formation of blood vessel within 2 to 4 weeks with significant antimicrobial effect and tissue regeneration have been reported using extended 3D scaffolds on a par with the traditional 2D scaffold.
Furthermore, for diabeti-related wound healing, Chen et al. [125] have developed 3D vertical/radially aligned nanofiber scaffolds in bone marrow mesenchymal stem cells (BMSC) transplant. It offers the advantage of shape-recovery upon compression in atmospheric and aquatic conditions fitting in for a variety of type 2 diabetic wounds. These BMSC embedded scaffold has potentially enhanced granulation, angiogenesis, and collagen deposition. In addition, they were also found to inhibit the formation of M1-type macrophage and pro-inflammatory cytokines IL-6 and TNF-α and thereby promoting M2-type macrophage and expression of IL-4 and IL-10.
In yet another study, Lv et al. [126] have reported on the wound healing property of PCL/gelatin nanofibrous scaffold containing silicate based bioceramic particles (NAGEL) fabricated using co-electrospinning process. The uniform distribution of bioceramic particles in the PCL-gelatin nanofibers aided Si ions in sustained release during their degradation. Significantly, they promoted cell adhesion, proliferation, and migration by activating epithelial / endothelial to mesenchymal transition pathway both in vitro and in vivo. Such synergistic effects of the functional biomaterials involving conductive nanocomposite scaffold have opened new vistas in wound healing. Similarly, Ren et al. [127] have reported PILA electrospun nanofiber impregnated with dimethyloxalylglycine-decorated mesoporous silica NPs for wound healing by accelerating the expression of human umbilical vein endothelial cells (HUVECs).
It is noteworthy to mention that several metal nanoparticles with antimicrobial potential are electrospun into nanofiber for counteracting multidrug-resistant bacteria and for wound-healing application. Yang et al. [128] have developed wound dressings using 6-aminopenicillanic acid decorated-gold nanoparticles to inhibit the growth of multidrug resistant (MDR) bacteria. These materials are then electrospun into PCL-gelatin fibers to challenge MDR strains in promoting faster wound-healing. Xi et al. [129] have developed an elastomeric, photoluminescent, biocompatible, and antimicrobial polypeptide based PCE-PCL nanofiber to inhibit MDR bacteria. There was also significant enhancement in skin-thickness wound healing and tissue regeneration in mouse model attributing for competitive multifunctional wound dressing. Some of the developed products patented using nanofiber technology has been shown in Table 5.
Plant based materials, besides their potential medicinal properties, pose great challenge toward electrospinnability and mechanical characteristics in fabricating them in the form of nanofibrous mats. The development of hybrid nanofibrous scaffold has opened new vistas in improving the mechanical properties and retaining the biological efficiency of plant based materials. For instance, Aloe vera gel was extracted and blended with two different polymers viz. gelatin and poly(ε-caprolactone) and spun into nanofibrous scaffold under optimized conditions. The electrospun nanofibers were found non-toxic with improved mechanical properties and hydrophilicity for extended application toward skin tissue engineering [130].
A similar study was conducted by Solaberrieta et al. [131], in which the bioactive components from Aloe vera skin extract were electrospun into nanofibers using poly(ethylene oxide) solutions. The successful encapsulation and incorporation into poly(ethylene oxide) were determined by changes in the nanofiber morphology with demonstrated bimodal diameter distributions. Besides decreased thermal stability, the encapsulation efficiency was found to be relatively high (92%, 76%, and 105%). These poly(ethylene oxide)-Aloe vera electrospun nanofibers with significant antioxidant activity were found to have their application in food packaging industry to help decrease oxidation process during storage of packaged food.
Ghorbani et al. [132] have fabricated Aloe vera-loaded nanofibrous scaffold encapsulating zein/polycaprolactone/collagen with the aid of ZnO (1% w) nanoparticles. The nanofibers developed had shown excellent thermal stability and mechanical properties with Aloe vera extract at 8% wt and zein/polycaprolactone at 70:30 ratio. Moreover, the nanofibrous scaffolds were found biocompatible and biodegradable upon enhancing the adhesion and proliferation properties of fibroblast cells. The potent antimicrobial activity of the fabricated scaffold was found to have its application toward wound healing by containing the growth of pathogenic bacteria.
Nanofibers in drug delivery
Electrospinning has enabled easy encapsulation of bioactive molecules/drugs and has prevented its loss facilitating sustained release to exhibit its maximum activity. The objective in delivering a predetermined dose of drug efficiently, specific to tissue/cell for a defined period of time, has been achieved using electrospinning for drug delivery applications. They have also been applied to treat various diseases via oral and topical routes of administration of poorly soluble or insoluble drugs. Typically, drugs that undergo rapid metabolism, extensive degradation, and with low solubility and instability (mostly anti-inflammatory and antioxidant drugs) have been electrospun into fibers for sustained release [133]. The electrospun nanofibers fabricated scaffolds are delivered either by viral or non-viral nucleic acids. Subsequently, immobilizing the drugs onto the nanofibers remains a great challenge and which is overcome by the most commonly adopted entrapment method. In case of nanofibers, the drugs are entrapped through the crosslinking of the polymeric fibers or by an intermediate carriers attributing for core-sheath encapsulation. For instance, alginate when crosslinked with calcium acts as common polymer for entrapment of drugs in bulk [134]. In core-sheath approach, polymers (PCL/PLGA) would encapsulate the drugs with BSA-dextran/chitosan core to confer stability [135].
Multidrug delivery, a novel approach comprising multiple drugs with or without similar remedial properties, has been electrospun into desirable polymers. Wang et al. [136] have developed a novel controlled drug release system using Chitosan NPs-PCL polymer electrospun fibers. Further, small molecule rhodamine B and naproxen have been successfully loaded in the core-sheath region for sustained release. In this line, MPEG-b-PLA micelles-chitosan-PEO has been electrospun with both hydrophobic and hydrophilic drugs viz. 5-FU and Cefradine. This model exhibited the final release proportion of about 91.4% prior to continuous exposure for 109 h. HepG-2 cells treated with the micellar-loaded nanofibers showed 45.9% viability prior to 3 days of exposure with 21.6 μg 5-FU [137]. With the core–shell nanofibers composed of PVA crosslinked PAN, water- and organic solvent-soluble drugs namely diclofenac sodium and gentamicin sulfate have been loaded successfully at concentrations 1–2% w/w PAN/GEN that enabled deep penetration of PAV/DS into the nanofibers [138].
Recently, Nagiah and co-workers have developed high tensile tripolymeric triaxial electrospun fibrous matrix for delivering multiple drugs. The polymers used were PCL as core and PLGA as sheath with an intermediate gelatin layer. They have demonstrated the dual release of small molecule rhodamine B and a model protein FITC-BSA incorporated in the sheath and the intermediate gelatin layers. They were able to support the adhesion, migration, and proliferation of mesenchymal stem cells. By this approach, the shrinkage encountered in conventional electrospinning technique was reduced with additive biomechanical stability [139]. Some of the polymeric nanofibers used for effective drug delivery have been enlisted in Table 6.
Conclusion and future outlook
After reviewing several techniques, forms, materials used in electrospinning for the generation of nanofiber materials, and their potential biomedical applications, a paradigm shift toward fabrication of 3D architecture constitutes one of the key elements for clinical implications. This is on a par with the conventional electrospun fiber mats produced by direct deposition of fibers onto the substrate. With 3D intervention, the solubility, low porosity, swelling, and collapsing of the 2D fibers hindering cell infiltration shall be overcome. As 3D nanofiber facilitates the typical in vivo settings for cells to adhere, proliferate, and differentiate within the matrix, their applications in tissue regeneration are the most promising. Moreover, these electrospun nanofibrous scaffolds have been much sought after approach in wound healing as a wound dresser. The typical alignment of the fibers helps fix the drug and release them in a controlled fashion thereby expending the process of healing. Furthermore, the increase in migration efficiency of cells seeded onto fibers helps greatly in tissue repair and regeneration. These advancements have been achieved with the thorough understanding of the spinneret, its control, and mechanism involved in the electrospinning for a better control suiting various applications. It is this understanding that had sown seeds to involve diverse materials including polymers, small molecules, nanoparticles, and colloids to be electrospun in to fibers. Besides innovation, technology transfer, expansion, and commercialization of post-electrospinning product remain the need of the hour. The list of commercialized products manufacturing using nanofiber technology has been enlisted in Table 7. One of the commercially successful products developed was nanofiber-based respiratory mask capable with a filtering efficiency up to 95%. This is in response to encounter COVID-19, where nano-filtered face mask was developed with nanofibers (in the diameter range of 100–500 nm) arranged in orthogonal/unidirectional directions to relieve the challenges. For instance, PVP/TiO2 nanofibers have been electrospun for potential application toward filtration and environmental remediation [140]. In addition, antimicrobial nanoparticles were also introduced into the nanofibers to increase the filtration efficacy and which is possible only through electrospinning.
There is no doubt that this technology enabled nanofibers would hit the global market by the end of 2022. In order to meet out the demand, there needs to be an increased scalability without compromising the quality, diversity, functionality, and environmental sustainability. The use of chemicals and their disposals need to be critically handled with regard to environmental safety. Studies pertaining to toxicity and environmental burden need to be conducted owing to address respiratory problems related to the inhalation of solvents/short electrospun nanofibers (Ag/inorganic) and to draw to a conclusion facilitating the clearance of nanofibers from the human system.
References
Tiwari JN, Tiwari RN, Kim KS (2012) Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured Mater for advanced electrochemical energy devices. Prog Mater Sci 57:724–803. https://doi.org/10.1016/j.pmatsci.2011.08.003
Yong KT, Yu S (2012) AlN nanowires: synthesis, physical properties and nanoelectronics applications. J Mater Sci 47:5341–5360. https://doi.org/10.1007/s10853-012-6388-0
Kenry, Lim CT (2013) Synthesis, optical properties, and chemical–biological sensing applications of one-dimensional inorganic semiconductor nanowires. Prog Mater Sci 58:705–48. https://doi.org/10.1016/j.pmatsci.2013.01.001
Visakh PM, Morlanes MJ (2016) Nanomaterials and nanocomposites: zero to three-dimensional materials and their composites. John Wiley & Sons, Germany
Liu H, Ding X, Zhou G, Li P, Wei X, Fan Y (2013) Electrospinning of nanofibers for tissue engineering applications. J Nanomater 2013. https://doi.org/10.1155/2013/495708
Horne J, McLoughlin L, Bridgers B, Wujcik EK (2020) Recent development in nanofiber-based sensors for disease detection, immunosensing and monitoring. Sensor Actuat Rep 2(1):100005. https://doi.org/10.1016/j.snr.2020.100005
Reneker DH, Chun I (1996) Nanometer diameter fibers of polymer, produced by electrospinning. Nanotechnol 1996(7):216–223. https://doi.org/10.1088/0957-4484/7/3/009
Behrens AM, Casey BJ, Sikorski MJ, Wu KL, Tutak W, Sandler AD et al (2014) In situ deposition of PLGA nanofibers via solution blow spinning. ACS Macro Lett 3:249–254. https://doi.org/10.1021/mz500049x
Shah S, Yin PT, Uehara TM, Chueng STD, Yang L, Lee KB (2014) Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv Mater 26:3673–3680. https://doi.org/10.1002/adma.201400523
Yang X, Zou W, Su Y, Zhu Y, Jiang H, Shen J et al (2014) Activated nitrogen-doped carbon nanofibers with hierarchical pore as efficient oxygen reduction reaction catalyst for microbial fuel cells. J Power Sources 266:36–42. https://doi.org/10.1016/j.jpowsour.2014.04.126
Aruchamy K, Mahto A, Nataraj SK (2018) Electrospun nanofibers, nanocomposites and characterization of art: insight on establishing fibers as product. Nano-Struct 16:45–58. https://doi.org/10.1016/j.nanoso.2018.03.013
Xue J, Tong Wu, Dai Y, Xia Y (2019) Electrospinning and electrospun nanofibers: methods, materials and applications. Chem Rev 119(8):5298–5415. https://doi.org/10.1021/acs.chemrev.8b00593
Pei B, Wang W, Fan Y, Wang X, Watari F, Li X (2017) Fiber-reinforced scaffolds in soft tissue engineering. Regen Biomater 4(4):257–268. https://doi.org/10.1093/rb/rbx021
Tamayol A, Akbari M, Annabi N, Paul A, Khademhosseini A, Juncker D (2013) Fiber-based tissue engineering: progress, challenges, and opportunities. Biotechnol Adv 31(5):669–687. https://doi.org/10.1016/j.biotechadv.2012.11.007
Akbari M, Tamayol A, Annabi N, Juncker D (2014) Chapter 1: microtechnologies in the fabrication of fibers for tissue engineering in microfluidics for medical applications. 1–18. https://doi.org/10.1039/9781849737593-00001. eISBN: 978–1–84973–759–3
Al-Hazeem NZA (2018) Nanofibers and electrospinning method, novel nanomaterials - synthesis and applications, George Z. Kyzas and Athanasios C. Mitropoulos, IntechOpen. https://doi.org/10.5772/intechopen.72060. Available from: https://www.intechopen.com/chapters/59431
Kiseleva AP, Krivoshapkin PV, Krivoshapkina EF (2020) Recent advances in development of functional spider silk-based hybrid materials. Front Chem 8:554. https://doi.org/10.3389/fchem.2020.00554
Mendes ISF, Prates A, Evtuguin DVE (2021) Production of rayon fibers from cellulosic pulps: state of the art and current developments. Carbohydr Polym 273:118466. https://doi.org/10.1016/j.carbpol.2021.118466
Mohammed L, Ansari MNM, Pua G, Jawaid M, Islam MS (2015) A review on natural fiber reinforced polymer composite and its applications. Int J Polym Sci. https://doi.org/10.1155/2015/243947
Boys CV (1887) On the production, properties, and some suggested uses of the finest threads. Proc Phys Soc London 9:8–19. https://doi.org/10.1088/1478-7814/9/1/303
Li D, Xia Y (2004) Electrospinning of nanofibers: reinventing the wheel? Adv Mater 16:1151–1170. https://doi.org/10.1002/adma.200400719
Xue J, Xie J, Liu W, Xia Y (2017) Electrospun nanofibers: new concepts, materials and applications. Acc Chem Res 50:1976–1987. https://doi.org/10.1021/acs.accounts.7b00218
Fridrikh SV, Yu JH, Brenner MP, Rutledge GC (2003) Controlling the fiber diameter during electrospinning. Phys Rev Lett 90(14):144502. https://doi.org/10.1103/PhysRevLett.90.144502
Ko FK (2004) Nanofiber technology: bridging the gap between nano and macro world. In: Guceri S, Gogotsi Y, Kuznetsov V (ed) NATO ASI on Nanoengineeered Nanofibrous Materials. NATO Series II, pp 169
Yamashita Y, Ko F, Tanaka A, Miyake H (2007) Characteristics of elastomeric nanofiber membranes produced by electrospinning. J Text Eng 53(4):137–142. https://doi.org/10.4188/jte.53.137
Verma SK, Jha E, Kiran KJ, Bhat S, Suar M, Mohanty PS (2016) Synthesis and characterization of novel polymer-hybrid silver nanoparticles and its biomedical study. Mater Today 3(6):1949–1957. https://doi.org/10.1016/j.matpr.2016.04.096
Zheng Y, Cao H, Zhou Z, Mei X, Yu L, Chen X, He G, Zhao Y, Wu D, Sun D (2019) Concentrated multi-nozzle electrospinning. Fibers Polym 20:1180–1186. https://doi.org/10.1007/s12221-019-8984-y
Qu H, Wei S, Guo Z (2013) Coaxial electrospun nanostructures and their applications. J Mater Chem A 1(38):11513–11528. https://doi.org/10.1039/C3TA12390A
Levorson EJ, Raman Sreerekha P, Chennazhi KP, Kasper FK, Nair SV, Mikos AG (2013) Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed Mater 8(1):014103. https://doi.org/10.1088/1748-6041/8/1/014103
Park S-H, Kim MS, Lee B, Park JH, Lee HJ, Lee NK, Jeon NL, Suh KY (2016) ACS Appl Mater Interfaces 8(4):2826–2832. https://doi.org/10.1021/acsami.5b11529
Kim BS, Park KE, Kim MH, You HK, Lee J, Park WH (2015) Effect of nanofiber content on bone regeneration of silk fibroin/poly(ε-caprolactone) nano/microfibrous composite scaffolds. Int J Nanomedicine 10:485–502. https://doi.org/10.2147/IJN.S72730
Kwak S, Haider A, Gupta KC, Kim S, Kang IK (2016) Micro/nano multilayered scaffolds of PLGA and collagen by alternately electrospinning for bone tissue engineering. Nanoscale Res Lett 11(1):323. https://doi.org/10.1186/s11671-016-1532-4
Leong MF, Rasheed MZ, Lim TC, Chian KS (2009) In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D, L-lactide) scaffold fabricated by cryogenic electrospinning technique. J Biomed Mater Res Part A 91A:231–240
Bulysheva AA, Bowlin GL, Petrova SP, Yeudall WA (2013) Enhanced chemoresistance of squamous carcinoma cells grown in 3D cryogenic electrospun scaffolds. Biomed Mater 8:055009
Medeiros ELG, Braz AL, Porto IJ, Menner A, Bismarck A, Boccaccini AR, Lepry WC, Nazhat SN, Medeiros ES, Blaker JJ (2016) Porous bioactive nanofibers via cryogenic solution blow spinning and their formation into 3D macroporous scaffolds. ACS Biomater Sci Eng 2:1442–1449
Li W, Shi L, Zhou K, Zhang X, Ullah I, Ou H, Zhang W, Wu T (2019) Facile fabrication of porous polymer fibers via cryogenic electrospinning system. J Mater Process Technol 266:551–557
Zaiss S, Brown TD, Reichert JC, Berner A (2016) Poly(ε-caprolactone) scaffolds fabricated by Melt electrospinning for bone tissue engineering. Mater (Basel) 9(4):232. https://doi.org/10.3390/ma9040232
Zhao YT, Zhang J, Gao Y, Liu X-F, Liu J-J, Wang X-X, Xiang H-F, Long Y-Z (2020) Self-powered portable melt electrospinning for in situ wound dressing. J Nanobiotechnol 18:111. https://doi.org/10.1186/s12951-020-00671-w
Großhaus C, Bakirci E, Berthel M, Hrynevich A, Kade JC, Hochleitner G, Groll J, Dalton PD (2020) Melt electrospinning of nanofibers from medical-grade poly(ε-caprolactone) with a modified nozzle. Small 16:2003471. https://doi.org/10.1002/smll.202003471
Bolle ECL, Nicdao D, Dalton PD, Dargaville TR (2021) Production of scaffolds using melt electrospinning writing and cell seeding. In: Rainer A, Moroni L (eds) Computer-Aided Tissue Engineering: Methods and Protocols. Springer, Humana, N.Y, pp 111–124
Lukas D, Sarkar A, Pokorny P (2008) Self-organization of jets in electrospinning from free liquid surface: a generalized approach. J Appl Phys 103:084309. https://doi.org/10.1063/1.2907967
Niu H, Lin T (2012) Fiber generators in needleless electrospinning. J Nanomater 1–13. https://doi.org/10.1155/2012/725950
Nieminen HJ, Laidmäe I, Salmi A, Rauhala T, Paulin T, Heinämäki J (2018) Haeggström Ultrasound-enhanced electrospinning. Sci Rep 8:4437. https://doi.org/10.1038/s41598-018-22124-z
Hakkarainen E, Kõrkjas A, Laidmäe I, Lust A, Semjonov K, Kogermann K, Nieminen HJ, Salmi A, Korhonen O, Haeggström E, Heinämäki J (2019) Comparison of traditional and ultrasound-enhanced electrospinning in fabricating nanofibrous drug delivery systems. Pharmaceutics 11(10):495. https://doi.org/10.3390/pharmaceutics11100495
Liao Y, Loh CH, Tian M, Wang R, Fane AG (2018) Progress in electrospun polymeric nanofibrous membranes for water treatment: fabrication, modification and applications. Prog Polym Sci 77:69–94. https://doi.org/10.1016/j.progpolymsci.2017.10.003
Brown TD, Dalton PD, Hutmacher DW (2016) Melt electrospinning today: an opportune time for an emerging polymer process. Prog Polym Sci 56:116–166. https://doi.org/10.1016/j.progpolymsci.2016.01.001
Bhattarai N, Zhang M (2007) Controlled synthesis and structural stability of alginate-based nanofibers. Nanotechnol 18(45):455601. https://doi.org/10.1088/0957-4484/18/45/455601
Ding F, Deng H, Du Y, Shi X, Wang O (2014) Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 6(16):9477–9493. https://doi.org/10.1039/C4NR02814G
Wang M, Roy AK, Webster TJ (2017) Development of chitosan/poly(vinyl alcohol) electrospun nanofibers for infection related wound healing. Front Physiol 7:683. https://doi.org/10.3389/fphys.2016.00683
Zhou Y, Dong Q, Yang H, Liu X, Yin X, Tao Y, Bai Z, Xu W (2017) Photocrosslinked maleilated chitosan/methacrylated poly (vinyl alcohol) bicomponent nanofibrous scaffolds for use as potential wound dressings. Carbohydr Polym 168:220–226. https://doi.org/10.1016/j.carbpol.2017.03.044
Alavarse AC, de Oliveira Silva FW, Colque JT, da Silva VM, Prieto T, Venancio EC, Bonvent JJ (2017) Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater Sci Eng C Mater Biol Appl 77:271–281. https://doi.org/10.1016/j.msec.2017.03.199
Ahmed R, Tariq M, Ali I, Asghar R, Noorunnisa Khanam P, Augustine R, Hasan A (2018) Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol 120(Pt A):385–393. https://doi.org/10.1016/j.ijbiomac.2018.08.057
Adeli H, Khorasani MT, Parvazinia M (2019) Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int J Biol Macromol 122:238–254. https://doi.org/10.1016/j.ijbiomac.2018.10.115
Brenner EK, Schiffman JD, Thompson EA, Toth LJ, Schauer CL (2012) Electrospinning of hyaluronic acid nanofibers from aqueous ammonium solutions. Carbohydr Polym 8(1):926–929. https://doi.org/10.1016/j.carbpol.2011.07.033
Eatemadi A, Daraee H, Zarghami N, Melat Yar H, Akbarzadeh A (2016) Nanofiber: synthesis and biomedical applications. Artif Cells Nanomed Biotechnol 44(1):111–121. https://doi.org/10.3109/21691401.2014.922568
Ma H, Chen G, Zhang J, Liu Y, Nie J, Ma G (2017) Facile fabrication of core–shell polyelectrolyte complexes nanofibers based on electric field induced phase separation. Polymer 110:80–86. https://doi.org/10.1016/j.polymer.2016.12.062
Malkin A, Semakov A, Skvortsov I, Zatonskikh P, Kulichikhin V, Subbotin A, Semenov A (2017) Spinnability of dilute polymer solutions Macromol 50:8231–8244. https://doi.org/10.1021/acs.macromol.7b00687
Vítková L, Musilová L, Achbergerová E, Minařík A, Smolka P, Wrzecionko E, Mráček A (2019) Electrospinning of Hyaluronan using polymer coelectrospinning and intermediate solvent. Polymers (Basel) 11(9):1517. https://doi.org/10.3390/polym11091517
Fuenteslópez CV, Ye H (2020) Electrospun fibres with hyaluronic acid-chitosan nanoparticles produced by a portable device. Nanomaterials (Basel) 10(10):2016. Published 2020 Oct 13. https://doi.org/10.3390/nano10102016
Lu WP, Guo Y (2018) Electrospinning of collagen and its derivatives for biomedical applications, Novel Aspects of Nanofibers, Tong Lin. IntechOpen. https://doi.org/10.5772/intechopen.73581
Deng A, Yang Y, Du S, Yang S (2018) Electrospinning of in situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater Sci 6(8):2197–2208
Türker E, Yildiz ÜH, Arslan YA (2019) Biomimetic hybrid scaffold consisting of co-electrospun collagen and PLLCL for 3D cell culture. Int J Biol Macromol 139:1054–1062. https://doi.org/10.1016/j.ijbiomac.2019.08.082
Li X, Li M, Sun J, Zhuang Y, Shi J, Guan D, Chen Y, Dai J (2016) Radially aligned electrospun fibers with continuous gradient of SDF1α for the guidance of neural stem cells. Small 12(36):5009–5018. https://doi.org/10.1002/smll.201601285
Guo S, He L, Yang R, Chen B, Xie X, Jiang B, Weidong T, Ding Y (2020) Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J Biomater Sci Polym Ed 31(2):155–168. https://doi.org/10.1080/09205063.2019.1680927
Alnaqbi MA, Al Blooshi AG, Greish YE (2020) Polyethylene and polyvinyl chloride-blended polystyrene nanofibrous sorbents and their application in the removal of various oil spills. Adv Polym Technol 4097520. https://doi.org/10.1155/2020/4097520
Zhao W, Li J, Jin K, Liu W, Qiu X, Li C (2015) Fabrication of functional PLGA-based electrospun scaffolds and their applications in biomedical engineering. Mater Sci Eng C 59:1181–1194. https://doi.org/10.1016/j.msec.2015.11.026
Casasola R, Thomas NL, Trybala A, Georgiadou S (2014) Electrospun poly lactic acid (PLA) fibres: effect of different solvent systems on fibre morphology and diameter. Polym 55(18):4728–4737. https://doi.org/10.1016/j.polymer.2014.06.032
Kanmaz D, Toprakcz HAK, Olmez H, Toprakci O (2018) Electrospun polylactic acid based nanofibers for biomedical applications. Mater Sci Res India 15:224–240. https://doi.org/10.13005/msri/150304
Ebnesajjad S (2012) Handbook of biopolymers and biodegradable plastics: properties, processing and applications. William Andrew
Yalcin E, Cicek F, Cavusoglu K (2014) Siklodekstrin Baglı Poli (Laktid-Ko-Glikolid) Mikro partikullerinin Sentezi. Karakterizasyonu, In vitro Kolesterol Gideriminde Kullanılabilirligi, Cumhuriyet Universitesi Fen-Edebiyat Fakultesi Fen Bilimleri Dergisi 35(3):9–22
González E, Shepherd LM, Saunders L, Frey MW (2016) Surface Functional Poly(lactic Acid) Electrospun Nanofibers for Biosensor Applications. Mater 9(1):47. https://doi.org/10.3390/ma9010047
Nicosia A, Gieparda W, Foksowicz-Flaczyk J, Walentowska J, Wesołek D, Vazquez B, Prodi F, Belosi F (2015) Air filtration and antimicrobial capabilities of electrospun PLA/PHB containing ionic liquid. Sep Purif Technol 154:154–160. https://doi.org/10.1016/j.seppur.2015.09.037
Rivers TJ, Hudson TW, Schmidt CE (2002) Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv Funct Mater 12:33–37. https://doi.org/10.1002/1616-3028
Shirakawa H, Louis EJ, MacDiarmid AG, Chiang CK, Heeger AJ (1977) Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene, (CH)x. J Chem Soc Chem Commun 578–580. https://doi.org/10.1039/C39770000578
Hong SY, Marynick DS (1992) Understanding the conformational stability and electronic structures of modified polymers based on polythiophene. Macromol 25:4652–4657. https://doi.org/10.1021/ma00044a029
Chronakis IS, Grapenson S, Jakob A (2006) Conductive polypyrrole nanofibers via electrospinning: electrical and morphological properties. Polym 47:1597–1603. https://doi.org/10.1016/j.polymer.2006.01.032
Wang L, Wu Y, Hu T, Guo B, Ma PX (2017) Electrospun conductive nanofibrous scaffolds for engineering cardiac tissue and 3D bioactuators. Acta Biomater 59:68–81. https://doi.org/10.1016/j.actbio.2017.06.036
Guo B, Ma PX (2018) Conducting polymers for tissue engineering. Biomacromol 19(6):1764–1782. https://doi.org/10.1021/acs.biomac.8b00276
Persano L, Dagdeviren C, Su Y et al (2013) High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat Commun 4:1633. https://doi.org/10.1038/ncomms2639
Abbasipour M, Khajavi R, Yousefi AA, Yazdanshenas ME, Razaghian F, Akbarzadeh A (2019) Improving piezoelectric and pyroelectric properties of electrospun PVDF nanofibers using nanofillers for energy harvesting application. Polym Adv Technol 30:279–291. https://doi.org/10.1002/pat.4463
Zhang Z, Hu J, Ma PX (2012) Nanofiber-based delivery of bioactive agents and stem cells to bone sites. Adv Drug Deliv Rev 64(12):1129–1141. https://doi.org/10.1016/j.addr.2012.04.008
McKay CS, Finn MG (2014) Click chemistry in complex mixtures: bioorthogonal bioconjugation. Chem Biol 21(9):1075–1101. https://doi.org/10.1016/j.chembiol.2014.09.002
Meng X, Edgar KJ (2016) Click reactions in polysaccharide modification. Prog Poly Sci 53:52–85. https://doi.org/10.1016/j.progpolymsci.2015.07.006
Saurer EM, Flessner RM, Buck ME, Lynn DM (2011) Fabrication of covalently crosslinked and amine-reactive microcapsules by reactive layer-by-layer assembly of azlactone-containing polymer multilayers on sacrificial microparticle templates. J Mater Chem 21:1736–1745. https://doi.org/10.1039/C0JM02633F
Broderick AH, Carter MCD, Lockett MR, Smith LM, Lynn DM (2013) Fabrication of oligonucleotide and protein arrays on rigid and flexible substrates coated with reactive polymer multilayers. ACS Appl Mater Interfaces 5(2):351–359. https://doi.org/10.1021/am302285n
Kratochvil MJ, Tal-Gan Y, Yang T, Blackwell HE, Lynn DM (2015) Nanoporous superhydrophobic coatings that promote the extended release of water-labile quorum sensing inhibitors and enable long-term modulation of quorum sensing in Staphylococcus aureus. ACS Biomater Sci Eng 1(10):1039–1049. https://doi.org/10.1021/acsbiomaterials.5b00313
Kratochvil MJ, Yang T, Blackwell HE, Lynn DM (2017) Nonwoven polymer nanofiber coatings that inhibit quorum sensing in Staphylococcus aureus: toward new nonbactericidal approaches to infection control. ACS Infect Dis 3(4):271–280. https://doi.org/10.1021/acsinfecdis.6b00173
Xu JF, Chen YZ, Wu D, Wu LZ, Tung CH, Yang QZ (2013) Photoresponsive hydrogen-bonded supramolecular polymers based on a stiff stilbene unit. Angew Chem Int Ed 52:9738–9742. https://doi.org/10.1002/anie.201303496
Coverdale BDM, Gough JE, Sampson WW, Hoyland JA (2017) Use of lecithin to control fiber morphology in electrospun poly (ε-caprolactone) scaffolds for improved tissue engineering applications. J Biomed Mater Res A 105(10):2865–2874. https://doi.org/10.1002/jbm.a.36139
Jorgensen L, Qvortrup K, Chronakis IS (2015) Phospholipid electrospun nanofibers: effect of solvents and co-axial processing on morphology and fiber diameter. RSC Adv 5:53644–53652. https://doi.org/10.1039/C5RA10498J
Cashion MP, Li X, Geng Y, Hunley MT, Long TE (2010) Gemini surfactant electrospun membranes. Langmuir 26(2):678–683. https://doi.org/10.1021/la902287b
Hemp ST, Hudson AG, Allen MH, Pole SS, Moore RB, Long TE (2014) Solution properties and electrospinning of phosphonium Gemini surfactants. Soft Matter 10:3970–3977. https://doi.org/10.1039/C4SM00271G
Nuansing W, Georgilis E, de Oliveira TVAG, Charalambidis G, Eleta A, Coutsolelos AG, Mitraki A, Bittner AM (2014) Electrospinning of tetraphenylporphyrin compounds into wires. Part Part Syst Charact 31:88–93. https://doi.org/10.1002/ppsc.201300293
Celebioglu A, Uyar T (2013) Electrospinning of nanofibers from non-polymeric systems: electrospun nanofibers from native cyclodextrins. J Colloid Interface Sci 404:1–7. https://doi.org/10.1016/j.jcis.2013.04.034
Topuz F, Uyar T (2018) Electrospinning of cyclodextrin functional nanofibers for drug delivery applications. Pharmaceutics 11(1):6. https://doi.org/10.3390/pharmaceutics11010006
Xiang S, Tang HW, Zhou J, Li XZ (2019) Electrospinning of hydroxypropyl-beta-cyclodextrin/polyvinylpyrrolidone resveratrol-loaded nanofibers: preparation and characterization. Indian J Pharm Sci 81(4):618–625. https://doi.org/10.36468/pharmaceutical-sciences.552
Choi SS, Lee SG, Im SS, Kim SH, Joo YL (2003) Silica nanofibers from electrospinning/sol-gel process. J Mater Sci Lett 22:891–893. https://doi.org/10.1023/A:1024475022937
Gu Y, Chen D, Jiao X (2005) Synthesis and electrochemical properties of nanostructured LiCoO2 fibers as cathode materials for lithium-ion batteries. J Phys Chem B 109:17901–17906. https://doi.org/10.1021/jp0521813
Li D, McCann JT, Xia Y, Marquez M (2006) Electrospinning: a simple and versatile technique for producing ceramic nanofibers and nanotubes. J Am Ceram Soc 89:1861–1869. https://doi.org/10.1002/9780470588246.ch47
Saquing CD, Manasco JL, Khan SA (2009) Electrospun nanoparticle–nanofiber composites via a one-step synthesis. Small 5(8):944–951. https://doi.org/10.1002/smll.200801273
Penchev H, Paneva D, Manolova N, Rashkov I (2010) Hybrid nanofibrous yarns based on N-carboxyethylchitosan and silver nanoparticles with antibacterial activity prepared by self-bundling electrospinning. Carbohydr Res 345(16):2374–2380. https://doi.org/10.1016/j.carres.2010.08.014
Shi Q, Vitchuli N, Nowak J, Noar J, Caldwell JM, Breidt F, Bourham M, McCord M, Zhang X (2011) One-step synthesis of silver nanoparticle-filled nylon 6 nanofibers and their antibacterial properties. J Mater Chem 21(28):10330–10335. https://doi.org/10.1039/C1JM11492A
Chen J, Yang P, Wang C et al (2011) Ag nanoparticles/PPV composite nanofibers with high and sensitive opto-electronic response. Nanoscale Res Lett 6:121. https://doi.org/10.1186/1556-276X-6-121
He JH, Kong HY, Yang RR, Dou H, Faraz N, Wang L, Feng C (2012) Review on fiber morphology obtained by bubble-electrospinning and blown bubble spinning. Therm Sci 16:1263. https://doi.org/10.2298/TSCI1205263H
Naderi H, Matin MM, Bahrami AR (2011) Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl 26(4):383–417. https://doi.org/10.1177/0885328211408946
Zhao P, Gu H, Mi H, Rao C, Fu J, Turng L-S (2018) Fabrication of scaffolds in tissue engineering: a review. Front Mech Eng 13(1):107–119. https://doi.org/10.1007/s11465-018-0496-8
Lelkes PI, Li M, Perets A, Mondrinos MJ, Guo Y, Chen X, MacDiarmid AG, Ko FK, Finck CM, Wei Y (2007) Designing intelligent polymeric scaffolds for tissue engineering: blending and co-electrospinning synthetic and natural polymers. In: Gdoutos EE (eds) Experimental Analysis of Nano and Engineering Materials and Structures. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6239-1_413
Kim J, Hwang T, Aguilar L, Park CH, Kim CS (2016) A controlled design of aligned and random nanofibers for 3D bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci Rep 6:23761. https://doi.org/10.1038/srep23761
Pant H, Pandeya D, Nam K, Baek W, Hong S, Kim H (2011) Photocatalytic andantibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J Hazard Mater 189:465–471. https://doi.org/10.1016/j.jhazmat.2011.02.062
Hwang SH, Song J, Jung Y, Kweon OY, Song H, Jang J (2011) Electrospun ZnO/TiO2 composite nanofibers as a bactericidal agent. Chem Commun 47:9164–9166. https://doi.org/10.1039/c1cc12872h
Haider A, Kwak S, Gupta KC, Kang I-K (2015) Antibacterial activity and cytocompatibility of PLGA/CuO hybrid nanofiber scaffolds prepared by electrospinning. J Nanomater 1–10. https://doi.org/10.1155/2015/832762
Wu T, Zhang J, Wang Y, Li D, Sun B, El-Hamshary H, Yin M, Mo X (2018a) Fabrication and preliminary study of a biomimetic tri-layer tubular graft based on fibers and fiber yarns for vascular tissue engineering. Mater Sci Eng C 82:121–129. https://doi.org/10.1016/j.msec.2017.08.072
Wu T, Zhang J, Wang Y, Sun B, Yin M, Bowlin GL, Mo X (2018b) Design and fabrication of a biomimetic vascular scaffold promoting in situ endothelialization and Tunica media regeneration. ACS Appl Bio Mater 1:833–844. https://doi.org/10.1021/acsabm.8b00269
Qiu X, Lee BLP, Ning X, Murthy N, Dong N, Li S (2017) End-point immobilization of heparin on plasma-treated surface of electrospun polycarbonate-urethane vascular graft. Acta Biomater 51:138–147. https://doi.org/10.1016/j.actbio.2017.01.012
Eilenberg M, Enayati M, Ehebruster D, Grasl C, Walter I, Messner B, Baudis S, Potzmann P, Kaun C, Podesser BK, Wojta J, Bergmeister H (2020) Long term evaluation of nanofibrous, bioabsorbable polycarbonate urethane grafts for small diameter vessel replacement in rodents. Eur J Vasc Endovasc Surg 59(4):643–652. https://doi.org/10.1016/j.ejvs.2019.11.004
Gupta P, Lorentz KL, Haskett DG, Cunnane EM, Ramaswamy AK, Weinbaum JS, Vorp DA, Mandal BB (2020) Bioresorbable silk grafts for small diameter vascular tissue engineering applications: In vitro and in vivo functional analysis. Acta Biomater 105:146–158. https://doi.org/10.1016/j.actbio.2020.01.020
Kobayashi M, Lei NY, Wang Q, Wu BM, Dunn JC (2015) Orthogonally oriented scaffolds with aligned fibers for engineering intestinal smooth muscle. Biomater 61:75–84. https://doi.org/10.1016/j.biomaterials.2015.05.023
Wang Q, Wang K, Solorzano-Vargas RS, Lin P-Y, Walthers CM, Thomas A-L, Martin MG, Dunn JCY (2018) Bioengineered intestinal muscularis complexes with long-term spontaneous and periodic contractions. Plos One 13(5):e0195315. https://doi.org/10.1371/journal.pone.0195315
Wang J, Windbergs M (2017) Functional electrospun fibers for the treatment of human skin wounds. Eur J Pharm Biopharm 119:283–299. https://doi.org/10.1016/j.ejpb.2017.07.001
Jun I, Han HS, Edwards JR, Jeon H (2018) Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci 19(3):745. https://doi.org/10.3390/ijms19030745
Sun L, Gao W, Fu X, Shi M, Xie W, Zhang W, Zhao F, Chen X (2018) Enhanced wound healing in diabetic rats by nanofibrous scaffolds mimicking the Basketweave pattern of collagen fibrils in native skin. Biomater Sci 6:340–349. https://doi.org/10.1039/c7bm00545h
Pal P, Srivas PK, Dadhich P, Das B, Maulik D, Dhara S (2017) Nano- /microfibrous cotton-wool-like 3D scaffold with core-shell architecture by emulsion electrospinning for skin tissue regeneration. ACS Biomater Sci Eng 3:3563–3575. https://doi.org/10.1021/acsbiomaterials.7b00681
Ma B, Xie J, Jiang J, Wu J (2014) Sandwich-type fiber scaffolds with square arrayed microwells and nanostructured cues as microskin grafts for skin regeneration. Biomater 35(2):630–641. https://doi.org/10.1016/j.biomaterials.2013.09.111
Jiang J, Chen S, Wang H, Carlson MA, Gombart AF, Xie J (2018) CO2-expanded nanofiber scaffolds maintain activity of encapsulated bioactive materials and promote cellular infiltration and positive host response. Acta Biomater 68:237–248. https://doi.org/10.1016/j.actbio.2017.12.018
Chen S, Wang H, Su Y, John JV, McCarthy A, Wong SL, Xie J (2020) Mesenchymal stem cell-laden, personalized 3D scaffolds with controlled structure and fiber alignment promote diabetic wound healing. Acta Biomater 108:153–167. https://doi.org/10.1016/j.actbio.2020.03.035
Lv F, Wang J, Xu P, Han Y, Ma H, Xu H, Chen S, Chang J, Ke Q, Liu M et al (2017) A conducive bioceramic/polymer composite biomaterial for diabetic wound healing. Acta Biomater 60:128–143. https://doi.org/10.1016/j.actbio.2017.07.020
Ren X, Han Y, Wang J, Jiang Y, Yi Z, Xu H, Ke Q (2018) An aligned porous electrospun fibrous membrane with controlled drug delivery - an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater 70:140–153. https://doi.org/10.1016/j.actbio.2018.02.010
Yang X, Yang J, Wang Le, Ran B, Jia Y, Zhang L, Yang G, Shao H, Jiang X (2017) Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. ACS Nano 11(6):5737–5745. https://doi.org/10.1021/acsnano.7b01240
Xi Y, Ge J, Guo Y, Lei B, Ma PX (2018) Biomimetic elastomeric polypeptide-based nanofibrous matrix for overcoming multidrug-resistant bacteria and enhancing full-thickness wound healing/skin regeneration. ACS Nano 12(11):10772–10784. https://doi.org/10.1021/acsnano.8b01152
Baghersad S, Hajir Bahrami S, Mohammadi MR, Mojtahedi MRM, Milan PB (2018) Development of biodegradable electrospun gelatin/aloe-vera/poly(ε-caprolactone) hybrid nanofibrous scaffold for application as skin substitutes. Mater Sci Eng C Mater Biol Appl 93:367–379. https://doi.org/10.1016/j.msec.2018.08.020
Solaberrieta I, Jiménez A, Cacciotti I, Carmen GM (2020) Encapsulation of bioactive compounds from Aloe vera agrowastes in electrospun Poly(Ethylene oxide) Nanofibers. Polymers 12:1323. https://doi.org/10.3390/polym12061323
Ghorbani M, Nezhad-Mokhtari P, Ramazani S (2020) Aloe vera loaded nanofibrous scaffold based on zein/polycaprolactone/collagen for wound healing. Int J Biol Macromol 153:921–930. https://doi.org/10.1016/j.ijbiomac.2020.03.036
Yun J, Im JS, Lee YS, Kim HI (2011) Electro-responsive transdermal drug delivery behavior of PVA/PAA/MWCNT nanofibers. Eur Polym J 47:1893–1902. https://doi.org/10.1016/j.eurpolymj.2011.07.024
Hrib J, Sirc J, Hobzova R, Hampejsova Z, Bosakova Z, Munzarova M, Michalek J (2015) Nanofibers for drug delivery - incorporation and release of model molecules, influence of molecular weight and polymer structure. Beilstein J Nanotechnol 6:1939–1945. https://doi.org/10.3762/bjnano.6.198
Katas H, Hussain Z, Awang SA (2013) Bovine serum albumin-loaded chitosan/dextran nanoparticles: preparation and evaluation of ex vivo colloidal stability in serum. J Nanomater 536291. https://doi.org/10.1155/2013/536291
Wang Y, Wang B, Qiao W, Yin T (2010) A novel controlled release drug delivery system for multiple drugs based on electrospun nanofibers containing nanoparticles. J Pharm Sci 99(12):4805–4811. https://doi.org/10.1002/jps.22189
Hu J, Zeng F, Wei J, Chen Y, Chen Y (2014) Novel controlled drug delivery system for multiple drugs based on electrospun nanofibers containing nanomicelles. J Biomater Sci Polym Ed 25(3):257–268. https://doi.org/10.1080/09205063.2013.852367
Kharaghani D, Gitigard P, Ohtani H. Kim KO, Ullah S, Saito Y, Khan MQ, Kim IS (2019) Design and characterization of dual drug delivery based on in-situ assembled PVA/PAN core-shell nanofibers for wound dressing application. Sci Rep 9:12640. https://doi.org/10.1038/s41598-019-49132-x
Nagiah N, Murdock CJ, Bhattacharjee M, Nair L, Laurencin T (2020) Development of tripolymeric triaxial electrospun fibrous matrices for dual drug delivery applications. Sci Rep 10:609. https://doi.org/10.1038/s41598-020-57412-0
Sharma A, Pathak D, Patil DS, Dhiman N, Bhullar V, Mahajan A (2021) Electrospun PVP/TiO2 nanofibers for filtration and possible protection from various viruses like COVID-19. Technol 9:89. https://doi.org/10.3390/technologies9040089
Kong M, Chen X, Xing K, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63. https://doi.org/10.1016/j.ijfoodmicro.2010.09.012
Powell HM, Boyce ST (2009) Fiber density of electrospun gelatin scaffolds regulates morphogenesis of dermal–epidermal skin substitutes. J Biomed Mater Res A 84(4):1078–1086. https://doi.org/10.1002/jbm.a.31498
Kim SE, Heo DN, Lee JB et al (2009) Electrospun gelatin/polyurethane blended nanofibers for wound healing. Biomed Mater 4(4):044106. https://doi.org/10.1088/1748-6041/4/4/044106
Vargas EAT, do Vale Baracho NC, de Brito J et al (2010) Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomater 6(3):1069–1078. https://doi.org/10.1016/j.actbio.2009.09.018
Li D, Wang Y, Xia Y (2003) Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett 3:1167–1171. https://doi.org/10.1021/nl0344256
Smit E, Bűttner U, Sanderson RD (2005) Continuous yarns from electrospun fibers. Polymer 46(8):2419–2423. https://doi.org/10.1016/j.polymer.2005.02.002
Reneker DH, Yarin AL, Fong H, Koombhongse S (2000) Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J Appl Phys 87:4531–4547. https://doi.org/10.1063/1.373532
Shin YM, Hohman MM, Brenner MP, Rutledge GC (2001) Experimental characterization of electrospinning: the electrically forced jet and instabilities. Polymer 42:09955–09967. https://doi.org/10.1016/S0032-3861(01)00540-7
Taylor GI (1964) Disintegration of water drops in an electric field. Proc R Soc London A 280:383–397. https://doi.org/10.2307/2415876
Taylor GI (1966) The force exerted by an electric field on a long cylindrical conductor. Proc R Soc London, Ser A 291:145−158. https://doi.org/10.1098/rspa.1966.0085
Taylor G (1969) Electrically driven jets. Proc R Soc London, Ser A 313:453−475. https://doi.org/10.1098/rspa.1969.0205
Cooley JF (1902) Apparatus for electrically dispersing fluids, vol 692. U.S. Pat. pp 631
Morton WJ (1902) Method of dispersing fluid, vol 705. U.S. Pat. pp 691
Nollet JAX (1748) Part of a letter from Abbè Nollet, of the Royal Academy of Sciences at Paris, to Martin Folkes, Concerning Electricity. Philos Trans 45:187–194. https://doi.org/10.1098/rstl.1748.0018
Gray SA (1731) Letter concerning the electricity of water, from Mr. Stephen Gray to Cromwell Mortimer. Philos Trans R Soc London 37:227–260. https://doi.org/10.1098/rstl.1731.0040
Gilbert WD (1958) Magnete. Courier, New York
Lawson C, Sivan M, Pokorny P, Stanishevsky A, Lukáš D (2016) Poly(ε-caprolactone) nanofibers for biomedical scaffolds by high-rate alternating current electrospinning. MRS Adv 1:1289–1294
Sasithorn N, Martinová L, Horakova J, Mongkholrattanasit R (2016) Fabrication of silk fibroin nanofibres by needleless electrospinning. In: Electrospinning-Material, Techniques, and Biomedical Applications. Intech: London, UK, pp 95–113
Sirc J, Hampejsova Z, Trnovska J, Kozlik P, Hrib J, Hobzova R, Zajicova A, Holan V, Bosakova Z (2017) Cyclosporine A loaded electrospun poly(d, l-lactic acid)/poly(ethylene glycol) nanofibers: drug carriers utilizable in local immunosuppression. Pharm Res 34:1391–1401
Böttjer R, Grothe T, Ehrmann A (2018) Functional nanofiber mats for medical and biotechnological applications. In: Narrow and Smart Textiles. Springer: Cham, Swizerland, pp 203–214
Hampejsova Z, Batek J, Sirc J, Hobzova R, Bosakova Z (2019) Polylactide/polyethylene glycol fibrous mats for local paclitaxel delivery: comparison of drug release into liquid medium and to HEMA-based hydrogel model. Mon Für Chem-Chem Mon 150:1691–1696
Kurecic M, Mohan T, Virant N, Maver U, Stergar J, Gradišnik L, Kleinschek KS, Hribernik S (2019) A green approach to obtain stable and hydrophilic cellulose-based electrospun nanofibrous substrates for sustained release of therapeutic molecules. RSC Adv 9:21288–21301
Manikandan S, Divyabharathi M, Tomas K, Pavel P, David L (2019) Production of poly (ε-caprolactone) antimicrobial nanofibers by needleless alternating current electrospinning. Mater Today Proc 17:1100–1104
Klicova M, Klapstova A, Chvojka J, Koprivova B, Jencova V, Horakova J (2020) Novel double-layered planar scaffold combining electrospun PCL fibers and PVA hydrogels with high shape integrity and water stability. Mater Lett 263:127281
Laidmäe I, Nieminen H, Salmi A, Paulin T, Rauhala T, Falck K, Yliruusi J, Heinämäki J, Haeggström E, Veski P (2018) Device and method to produce nanofibers and constructs thereof. U.S. Patent 15/561,058, 15 March 2018
Zhou Y, Yang D, Chen X et al (2008) Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromol 9(1):349–354. https://doi.org/10.1021/bm7009015
Rafienia M, Saberi A, Poorazizi E (2017) A novel fabrication of PVA/alginate – bioglass electrospun for biomedical engineering application. Nanomed J 4(3):152–163. https://doi.org/10.22038/NMJ.2017.8956
Chutipakdeevong J, Ruktanonchai UR, Supaphol P (2013) Process optimization of electrospun silk fibroin fiber mat for accelerated wound healing. J Appl Polym Sci 130(5):3634–3644. https://doi.org/10.1002/app.39611
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28(1):142–150. https://doi.org/10.1016/j.biotechadv.2009.11.001
Chen JP, Chang GY, Chen JK (2008) Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf A Physicochem Eng Asp 313–314. https://doi.org/10.1016/j.colsurfa.2007.04.129
Lin J, Li C, Zhao Y et al (2012) Co-electrospun nanofibrous membranes of collagen and zein for wound healing. ACS Appl Mater Interfaces 4(2):1050–1057. https://doi.org/10.1021/am201669z
Konwarh R, Karak N, Misra M (2013) Electrospun cellulose acetate nanofibers: the present status and gamut of biotechnological applications. Biotechnol Adv 31(4):421–437. https://doi.org/10.1016/j.biotechadv.2013.01.002
Jannesari M, Varshosaz J, Morshed M, Zamani M (2011) Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomed 6:993–1003. https://doi.org/10.2147/IJN.S17595
Matthews JA, Wnek GE, Simpson DG et al (2002) Electrospinning of collagen nanofibers. Biomacromol 3(2):232–238. https://doi.org/10.1021/bm015533u
Rho KS, Jeong L, Lee G et al (2006) Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomater 27(8):1452–1461. https://doi.org/10.1016/j.biomaterials.2005.08.004
Liu SJ, Kau YC, Chou CY et al (2010) Electrospun PLGA/collagen nanofibrous membrane as early-stage wound dressing. J Membr Sci 355(1–2):53–59. https://doi.org/10.1016/j.memsci.2010.03.012
Neal RA, McClugage SG III, Link MC et al (2008) Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng Part C Methods 15(1):11–21. https://doi.org/10.1089/ten.tec.2007.0366
Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK (2002) Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res 60:613–621. https://doi.org/10.1002/jbm.10167
Yoshimoto H, Shin YM, Terai H, Vacanti JP (2003) A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomater 24:2077–2082. https://doi.org/10.1016/S0142-9612(02)00635-X
Shields KJ, Beckman MJ, Bowlin GL, Wayne JS (2004) Mechanical properties and cellular proliferation of electrospun collagen type II. Tissue Eng 10:1510–1517. https://doi.org/10.1089/ten.2004.10.1510
Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW (2005) Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomater 26:1261–1270. https://doi.org/10.1016/j.biomaterials.2004.04.037
Stitzel J, Liu J, Lee SJ, Komura M, Berry J, Soker S et al (2006) Controlled fabrication of a biological vascular substitute. Biomater 27:1088–1094. https://doi.org/10.1016/j.biomaterials.2005.07.048
Kim JH, Choung PH, Kim IY, Lim KT, Son HM, Choung YH, Cho CS, Chung JH (2009) Electrospun nanofibers composed of poly(ε-caprolactone) and polyethylenimine for tissue engineering applications. Mater Sci Eng C 29:1725–1731. https://doi.org/10.1016/j.msec.2009.01.023
Xu X-Y, Li X-T, Peng S-W, Xiao J-F, Liu C, Fang G, Chen KC, Chen G-Q (2010) The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomater 31(14):3967–3975. https://doi.org/10.1016/j.biomaterials.2010.01.132
Leung GKK, Wang YC, Wu W (2012) Peptide nanofiber scaffold for brain tissue reconstruction. In: Düzgüneş N (eds) Meth Enzymol, vol 508. pp 177–190. https://doi.org/10.1016/B978-0-12-391860-4.00009-4
Song J, Gao H, Zhu G, Cao X, Shi X, Wang Y (2015) The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 95:1039–1050. https://doi.org/10.1016/j.carbon.2015.09.011
Entekhabi E, Nazarpak MH, Moztarzadeh F, Sadeghi A (2016) Design and manufacture of neural tissue engineering scaffolds using hyaluronic acid and polycaprolactone nanofibers with controlled porosity. Mater Sci Eng: C 69:380–387. https://doi.org/10.1016/j.msec.2016.06.078
Li Y, Wang Y, Ye J, Yuan J, Xiao Y (2016) Fabrication of poly(ε-caprolactone)/keratin nanofibrous mats as a potential scaffold for vascular tissue engineering. Mater Sci Eng C Mater Biol Appl 68:177–183. https://doi.org/10.1016/j.msec.2016.05.117
Qian Y, Song J, Zhao X, Chen W, Ouyang Y, Yuan W, Fan C (2018) 3D fabrication with integration molding of a graphene oxide/polycarprolactone nanoscafold for neurite regeneration and angiogenesis. Adv Sci 5:1700499. https://doi.org/10.1002/advs.545
Fouad H, Basheer Al-S, Md Fayez A, Randa Al-F, Amer M (2019) Modified Bi-layered polycaprolactone nanofiber scaffolds for vascular tissue engineering applications. Nanosci Nanotechnol Lett 11(1):1–10. https://doi.org/10.1166/nnl.2019.2858
Zhang S, Chen L, Jiang Y, Cai Y, Xu G, Tong T, Zhang W, Wang L, Ji J, Shi P, Ouyang HW (2013) Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater 9(7):7236–7247. https://doi.org/10.1016/j.actbio.2013.04.003
Wang L, Wu Y, Guo B, Ma PX (2015) Nanofiber Yarn / Hydrogel core-shell scaffolds mimicking Native skeletal muscle tissue for guiding 3D Myoblast alignment, elongation and differentiation. ACS Nano 9(9):9167–9179. https://doi.org/10.1021/acsnano.5b03644
Chen W, Chen S, Morsi Y et al (2016) Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl Mater Interfaces 8(37):24415–24425
Sadat-Shojai M, Khorasani M-T, Jamshidi A (2016) A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem Eng J 289:38–47. https://doi.org/10.1016/j.cej.2015.12.079
Deepthi S, Nivedhitha Sundaram M, Deepti Kadavan J, Jayakumar R (2016) Layered chitosan-collagen hydrogel/aligned PLLA nanofiber construct for flexor tendon regeneration. Carbohydr Polym 153:492–500. https://doi.org/10.1016/j.carbpol.2016.07.124
Lin HY, Tsai WC, Chang SH (2017) Collagen-PVA aligned nanofiber on collagen sponge as bi-layered scaffold for surface cartilage repair. J Biomater Sci Polym Ed 28(7):664–678. https://doi.org/10.1080/09205063.2017.1295507
Gao S, Chen M, Wang P, Li Y, Yuan Z, Guo W, Zhang Z, Zhang X, Jing X, Li X, Liu S, Sui X, Xi T, Guo Q (2018) An electrospun fiber reinforced scaffold promotes total meniscus regeneration in rabbit meniscectomy model. Acta Biomater 73:127–140. https://doi.org/10.1016/j.actbio.2018.04.012
Lam LRW, Schilling K, Romas S, Misra R, Zhou Z, Caton JG, Zhang X (2021) Electrospun core-shell nanofibers with encapsulated enamel matrix derivative for guided periodontal tissue regeneration. Dent Mater J 40(5):1208–1216. https://doi.org/10.4012/dmj.2020-412
Khampieng T, Wnek GE, Supaphol P (2014) Electrospun DOXY-h loaded-poly(acrylic acid) nanofiber mats: In vitro drug release and antibacterial properties investigation. J Biomater Sci Polym Ed 25:1292–1305. https://doi.org/10.1080/09205063.2014.929431
Wang X, Yu DG, Li XY, Bligh SWA, Williams GR (2015) Electrospun medicated shellac nanofibers for colon-targeted drug delivery. Int J Pharm 490:384–390. https://doi.org/10.1016/j.ijpharm.2015.05.077
Paaver U, Heinämäki J, Laidmäe I, Lust A, Kozlova J, Sillaste E, Kirsimäe K, Veski P, Kogermann K (2015) Electrospun nanofibers as a potential controlled-release solid dispersion system for poorly water-soluble drugs. Int J Pharm 479:252–260. https://doi.org/10.1016/j.ijpharm.2014.12.024
Mu C, Wu Q (2017) Electrospun Poly(ε-caprolactone) Composite nanofibers with controlled release of Cis-diamminediiodoplatinum for a higher anticancer activity, Nanoscale Res Lett 12. https://doi.org/10.1186/s11671-017-2092-y
Kataria K, Gupta A, Rath G, Mathur RB, Dhakate SR (2014) In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int J Pharm 469:102–110. https://doi.org/10.1016/j.ijpharm.2014.04.047
Zhu X, Ni S, Xia T, Yao Q, Li H, Wang B, Wang J, Li X, Su W (2015) Anti-neoplastic cytotoxicity of SN-38-loaded PCL/Gelatin electrospun composite nanofiber scaffolds against human glioblastoma cells in vitro. J Pharm Sci 104:4345–4354. https://doi.org/10.1002/jps.24684
Aggarwal U, Goyal AK, Rath G (2017) Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer. Mater Sci Eng C 75:125–132. https://doi.org/10.1016/j.msec.2017.02.013
Esmaeili A, Haseli M (2017) Electrospinning of thermoplastic carboxymethyl cellulose/poly(ethylene oxide) nanofibers for use in drug-release systems. Mater Sci Eng C 77:1117–1127. https://doi.org/10.1016/j.msec.2017.03.252
Vashisth P, Raghuwanshi N, Srivastava AK, Singh H, Nagar H, Pruthi V (2017) Ofloxacin loaded gellan/PVA nanofibers - synthesis, characterization and evaluation of their gastroretentive/mucoadhesive drug delivery potential. Mater Sci Eng C 71:611–619. https://doi.org/10.1016/j.msec.2016.10.051
Dadras Chomachayi M, Solouk A, Akbari S, Sadeghi D, Mirahmadi F, Mirzadeh H (2018) Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: thyme essential oil and doxycycline monohydrate release study. J Biomed Mater Res-Part A 106:1092–1103. https://doi.org/10.1002/jbm.a.36303
Meng J, Agrahari V, Ezoulin MJ, Zhang C, Purohit SS, Molteni A, Dim D, Oyler NA, Youan BBC (2016) Tenofovir containing thiolated chitosan core/shell nanofibers: in vitro and in vivo evaluations. Mol Pharm 13:4129–4140. https://doi.org/10.1021/acs.molpharmaceut.6b00739
Sultanova Z, Kaleli G, Kabay G, Mutlu M (2016) Controlled release of a hydrophilic drug from coaxially electrospun polycaprolactone nanofibers. Int J Pharm 505:133–138. https://doi.org/10.1016/j.ijpharm.2016.03.032
Aytac Z, Uyar T (2017) Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: enhanced water solubility and slow release of curcumin. Int J Pharm 518:177–184. https://doi.org/10.1016/j.ijpharm.2016.12.061
Song W, Seta J, Chen L, Bergum C (2017) Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus aureus infection. Biomed Mater 12
Ping H, Quan Z, Yao G (2018) Dual drug loaded coaxial electrospun PLGA/PVP fiber for guided tissue regeneration under control of infection. Mater Sci Eng C 90:549–556. https://doi.org/10.1016/j.msec.2018.04.014
Acknowledgements
The author would like to thank the contributors and their articles which served as a base for preparing this manuscript. The timely support rendered by Saveetha Institute of Medical and Technical Sciences is greatly acknowledged. The author would like to acknowledge DST-SERB for grant-in-support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muthukrishnan, L. An overview on electrospinning and its advancement toward hard and soft tissue engineering applications. Colloid Polym Sci 300, 875–901 (2022). https://doi.org/10.1007/s00396-022-04997-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-022-04997-9