Abstract
Purpose
This study was conducted to investigate whether aspartate (Asp) could alleviate Escherichia coli lipopolysaccharide (LPS)-induced intestinal injury by modulating intestine inflammatory response.
Methods
Twenty-four weaned piglets were divided into four treatments: (1) non-challenged control; (2) LPS-challenged control; (3) LPS + 0.5 % Asp; and (4) LPS + 1.0 % Asp. After feeding with control, 0.5 or 1.0 % Asp-supplemented diets for 21 days, pigs were injected intraperitoneally with saline or LPS. At 4 h postinjection, blood and intestine samples were obtained.
Results
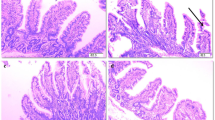
Asp supplementation to LPS-challenged pigs improved intestinal morphology, indicated by higher jejunal and ileal villus height/crypt depth ratio and lower ileal crypt depth linearly or quadratically. Asp also improved intestinal barrier function, indicated by increased jejunal and ileal diamine oxidase activities as well as enhanced protein expression of jejunal claudin-1 linearly or quadratically. In addition, Asp decreased plasma, jejunal and ileal tumor necrosis factor-α concentration and ileal caspase-3 protein expression linearly and quadratically. Moreover, Asp down-regulated the mRNA expression of toll-like receptor 4 (TLR4) and nucleotide-binding oligomerization domain protein (NOD) signaling-related genes, nuclear factor-κB (NF-κB) p65 and p38, decreased phosphorylation of jejunal p38, and increased phosphorylation of ileal extracellular signal-related kinase 1/2 linearly or quadratically. Finally, Asp increased mRNA expressions of TLR4 and NOD signaling negative regulators including radioprotective 105, suppressor of cytokine signaling 1, toll-interacting protein, Erbb2 interacting protein and centaurin β1 linearly or quadratically.
Conclusions
These results indicate that Asp supplementation is associated with inhibition of TLR4 and NODs/NF-κB and p38 signaling pathways and concomitant improvement of intestinal integrity under an inflammatory condition.



Similar content being viewed by others
References
Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J (2007) Restoration of barrier function in injured intestinal mucosa. Physiol Rev 87:545–564
Mitsui T, Fukatsu K, Yanagawa M, Amenomori S, Ogawa E, Fukuda T, Murakoshi S, Moriya T, Yasuhara H, Seto Y (2014) Truncal vagotomy temporarily decreases the pro- and anti-inflammatory cytokine levels in the small intestine. Surg Today 44:1123–1127
Wu G, Bazer FW, Davis TA, Jaeger LA, Johnson GA, Kim SW, Knabe DA, Meininger CJ, Spencer TE, Yin Y (2007) Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci 112:8–22
Flynn NE, Knabe DA, Mallick BK, Wu G (2000) Postnatal changes of plasma amino acids in suckling pigs. J Anim Sci 78:2369–2375
Pi D, Liu Y, Shi H, Li S, Odle J, Lin X, Zhu H, Chen F, Hou Y, Leng W (2014) Dietary supplementation of Aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J Nutr Biochem 25:456–462
Li P, Yin YL, Li D, Kim SW, Wu G (2007) Amino acids and immune function. Br J Nutr 98:237–252
Wu GY, Brosnan JT (1992) Macrophages can convert citrulline into arginine. Biochem J 281:45–48
Sukhotnik I, Helou H, Mogilner J, Lurie M, Bernsteyn A, Coran AG, Shiloni E (2005) Oral arginine improves intestinal recovery following ischemia-reperfusion injury in rat. Pediatr Surg Int 21:191–196
Zhou X, Wu X, Yin Y, Zhang C, He L (2012) Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids 43:813–821
Fukata M, Vamadevan AS, Abreu MT (2009) Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol 21:242–253
Uddin MJ, Kaewmala K, Tesfaye D, Tholen E, Looft C, Hoelker M, Schellander K, Cinar MU (2013) Expression patterns of porcine Toll-like receptors family set of genes (TLR1-10) in gut-associated lymphoid tissues alter with age. Res Vet Sci 95:92–102
Parlato M, Yeretssian G (2014) NOD-like receptors in intestinal homeostasis and epithelial tissue repair. Int J Mol Sci 15:9594–9627
Becker CE, O’Neill LA (2007) Inflammasomes in inflammatory disorders: the role of TLRs and their interactions with NLRs. Semin Immunopathol 29:239–248
Xu Y, Liu XD, Gong X, Eissa NT (2008) Signaling pathway of autophagy associated with innate immunity. Autophagy 4:110–112
Inohara Chamaillard, McDonald C, Nuñez G (2005) NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355–383
Doyle A, Zhang G, Abdel Fattah EA, Eissa NT, Li YP (2011) Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy-lysosome pathways. FASEB J 25:99–110
Liu Y, Huang J, Hou Y, Zhu H, Zhao S, Ding B, Yin Y, Yi G, Shi J, Fan W (2008) Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr 100:552–560
Mair KH, Sedlak C, Käser T, Pasternak A, Levast B, Gerner W, Saalmüller A, Summerfield A, Gerdts V, Wilson HL, Meurens F (2014) The porcine innate immune system: an update. Dev Comp Immunol 45:321–343
NRC (1998) Nutrient requirements of swine. 10th edn. National Academic Press, Washington
Shi HF, Liu YL, Li S, Zhu HL, Cheng F, Hou YQ, Ding BY, Pi DA, Leng WB (2013) Effect of Aspartic acid on growth performance, blood cell differential count, and blood biochemical measurements of weaned piglets after lipopolysaccharide challenge. China J Anim Sci 7:38–43
Liu YL, Li DF, Gong LM, Yi GF, Gaines AM, Carroll JA (2003) Effects of fish oil supplementation on the performance and the immunological, adrenal, and somatotropic responses of weaned pigs after an Escherichia coli lipopolysaccharide challenge. J Anim Sci 81:2758–2765
Mercer DW, Smith GS, Cross JM, Russell DH, Chang L, Cacioppo J (1996) Effects of lipopolysaccharide on intestinal injury: potential role of nitricoxide and lipid peroxidation. J Surg Res 63:185–192
Luna LG (1968) Manual of histologic staining methods of the armed forces institute of pathology. In: McGraw-Hill Book Company, 3rd edn. New York, pp 258
Liu YL, Han J, Huang JJ, Wang XQ, Wang FL, Wang JJ (2009) Dietary l-arginine supplementation improves intestinal function in weaned pigs after an Escherichia coli lipopolysaccharide challenge. Asian-Aust J Anim Sci 22:1667–1675
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Xun W, Shi L, Zhou H, Hou G, Cao T, Zhao C (2015) Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int Immunopharmacol 27:46–52
Peng X, Yan H, You Z, Wang P, Wang S (2004) Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 30:135–139
Anderson JM, Van Itallie CM (2009) Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1:a002584
Fukudome I, Kobayashi M, Dabanaka K, Maeda H, Okamoto K, Okabayashi T, Baba R, Kumagai N, Oba K, Fujita M, Hanazaki K (2014) Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol 47:100–107
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Tan B, Yin Y, Kong X, Li P, Li X, Gao H, Li X, Huang R, Wu G (2010) L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Hou Y, Wang L, Zhang W, Yang Z, Ding B, Zhu H, Liu Y, Qiu Y, Yin Y, Wu G (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Al-Sayeqh AF, Loughlin MF, Dillon E, Mellits KH, Connerton IF (2010) Campylobacter jejuni activates NF-kappaB independently of TLR2, TLR4, Nod1 and Nod2 receptors. Microb Pathog 49:294–304
Sabroe I, Parker LC, Dower SK, Whyte MK (2008) The role of TLR activation in inflammation. J Pathol 214:126–135
Chen Y, Chen D, Tian G, He J, Mao X, Mao Q, Yu B (2012) Dietary arginine supplementation alleviates immune challenge induced by Salmonella enterica serovar Choleraesuis bacterin potentially through the Toll-like receptor 4-myeloid differentiation factor 88 signalling pathway in weaned piglets. Br J Nutr 108:1069–1076
Coll RC, O’Neill LA (2010) New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun 2:406–421
Zhang G, Ghosh S (2002) Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem 277:7059–7065
Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, Kurt-Jones EA, Karp CL (2005) Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat Immunol 6:571–578
Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol 7:148–155
McDonald C, Chen FF, Ollendorff V, Ogura Y, Marchetto S, Lécine P, Borg JP, Nuñez G (2005) A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J Biol Chem 280:40301–40309
Eitel J, Krüll M, Hocke AC, N’Guessan PD, Zahlten J, Schmeck B, Slevogt H, Hippenstiel S, Suttorp N, Opitz B (2008) Beta-PIX and Rac1 GTPase mediate trafficking and negative regulation of NOD2. J Immunol 181:2664–2671
Kondo T, Kawai T, Akira S (2012) Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol 33:449–458
Ren W, Chen S, Yin J, Duan J, Li T, Liu G, Feng Z, Tan B, Yin Y, Wu G (2014) Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr 144:988–995
Anderson L, Seilhamer J (1997) A comparison of selected mRNA and protein abundances in human liver. Electrophoresis 18:533–537
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31422053 and 31372318), and the Project of the Hubei Provincial Department of Education (T201508).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Liu, Y., Shi, H. et al. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-κB and p38 signaling in weaned pigs after LPS challenge. Eur J Nutr 56, 1433–1443 (2017). https://doi.org/10.1007/s00394-016-1189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1189-x