Abstract
Purpose
Despite the detailed knowledge of the absorption and incorporation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) into plasma lipids and red blood cells (RBC) in humans, very little is known about docosapentaenoic acid (DPA, 22:5 n-3). The aim of this study was to investigate the uptake and incorporation of pure DPA and EPA into human plasma and RBC lipids.
Methods
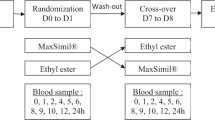
Ten female participants received 8 g of pure DPA or pure EPA in randomized crossover double-blinded manner over a 7-day period. The placebo treatment was olive oil. Blood samples were collected at days zero, four and seven, following which the plasma and RBC were separated and used for the analysis of fatty acids.
Results
Supplementation with DPA significantly increased the proportions of DPA in the plasma phospholipids (PL) (by twofold) and triacylglycerol (TAG) fractions (by 2.3-fold, day 4). DPA supplementation also significantly increased the proportions of EPA in TAG (by 3.1-fold, day 4) and cholesterol ester (CE) fractions (by 2.0-fold, day 7) and of DHA in TAG fraction (by 3.1-fold, day 4). DPA proportions in RBC PL did not change following supplementation. Supplementation with EPA significantly increased the proportion of EPA in the plasma CE and PL fractions, (both by 2.7-fold, day 4 and day 7) and in the RBC PL (by 1.9-fold, day 4 and day 7). EPA supplementation did not alter the proportions of DPA or DHA in any lipid fraction. These results showed that within day 4 of supplementation, DPA and EPA demonstrated different and specific incorporation patterns.
Conclusion
The results of this short-term study suggest that DPA may act as a reservoir of the major long-chain n-3 fatty acids (LC n-3 PUFA) in humans.




Similar content being viewed by others
References
Saravanan P, Davidson NC, Schmidt EB, Calder PC (2010) Cardiovascular effects of marine omega-3 fatty acids. Lancet 376(9740):540–550
Calder PC, Yaqoob P (2010) Omega-3 (n-3) fatty acids, cardiovascular disease and stability of atherosclerotic plaques. Cell Mol Biol (Noisy-le-grand) 56(1):28–37
Mann NJ, Johnson LG, Warrick GE, Sinclair AJ (1995) The arachidonic acid content of the Australian diet is lower than previously estimated. J Nutr 125(10):2528–2535
Or-Rashid MM, Odongo NE, Wright TC, McBride BW (2009) Fatty acid profile of bovine milk naturally enhanced with docosahexaenoic acid. J Agric Food Chem 57(4):1366–1371
Howe P, Meyer B, Record S, Baghurst K (2006) Dietary intake of long-chain omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition 22(1):47–53
Akiba S, Murata T, Kitatani K, Sato T (2000) Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biological Pharmacol 23(11):1293–1297
Kaur G, Sinclair AJ, Cameron-Smith D, Barr DP, Molero-Navajas JC, Konstantopoulos N (2011) Docosapentaenoic acid (22:5n–3) down-regulates the expression of genes involved in fat synthesis in liver cells. Prostaglandins Leukot Essent Fatty Acids 85(3–4):155–161
Yoshida H, Mawatari M, Ikeda I, Imaizumi K, Seto A, Tsuji H (1999) Effect of dietary seal and fish oils on triacylglycerol metabolism in rats. J Nutr Sci Vitaminol 45(4):411–421
Akiba S, Murata T, Kitatani K, Sato T (2000) Involvement of lipoxygenase pathway in docosapentaenoic acid-induced inhibition of platelet aggregation. Biol Pharm Bull 23(11):1293–1297
Phang M, Garg ML, Sinclair AJ (2009) Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific-redefining platelet response to fish oils. Prostaglandins Leukot Essent Fatty Acids 81(1):35–40
Gotoh N, Nagao K, Onoda S, Shirouchi B, Furuya K, Nagai T et al (2009) Effects of three different highly purified n-3 series highly unsaturated fatty acids on lipid metabolism in C57BL/KsJ-db/db mice. J Agric Food Chem 57(22):11047–11054
Pawar A, Jump DB (2003) Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatocytes. J Biol Chem 278(38):35931–35939
Kishida E, Tajiri M, Masuzawa Y (2006) Docosahexaenoic acid enrichment can reduce L929 cell necrosis induced by tumour necrosis factor. Biochim Biophys Acta 1761(4):454–462
Holub BJ, Swidinsky P, Park E (2011) Oral docosapentaenoic acid (22:5n–3) is differentially incorporated into phospholipid pools and differentially metabolized to eicosapentaenoic acid in tissues from young rats. Lipids [Research Support, Non-US Gov’t] 46(5):399–407
Kaur G, Begg DP, Barr D, Garg M, Cameron-Smith D, Sinclair AJ (2010) Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr [Research Support, Non-US Gov’t] 103(1):32–37
Meyer B, Mann N (2009) Comparison of seal oil to tuna oil on plasma lipid levels and blood pressure in hypertriglyceridaemic subjects. Lipids 44(9):827–835
Sullivan B, Brown J, Williams P, Meyer B (2008) Dietary validation of a new food frequency questionnaire that estimates long-chain omega-3 polyunsaturated fatty acids. Br J Nutr 99:660–666
Sullivan B, Williams P, Meyer B (2006) Biomarker validation of a new food frequency questionnaire that estimates long-chain omega-3 polyunsaturated fatty acids. Lipids 41:845–850
Swierk M, Williams P, Meyer B, Wilcox J, Russel K (2011) Validation of an Australian electronic food frequency questionnaire to measure polyunsaturated fatty acid intake. Nutrition 6:641–646
Sinclair A, O’Dea K, Dunstan G, Ireland P, Niall M (1987) Effects on plasma lipids and fatty acid composition of very low fat diets enriched with fish or kangaroo meat. Lipids 22:523–529
Ackman RG (2002) The gas chomatograph in practical analyses of common and uncommon fatty acids for the 21st century. Anal Chim Acta 465(1–2):175–192
Folch J, Lees M, Sloane-Stanley GH (1957) Methods for the isolation and purification of total lipids from animal tissue. J Biol Chem 226:497–509
Armstrong JM, Metherel AH, Stark KD (2008) Direct microwave transesterification of fingertip prick blood samples for fatty acid determinations. Lipids 43(2):187–196
Popovic T, Borozan S, Arsic A, Martacic JD, Vucic V, Trbovic A et al (2011) Fish oil supplementation improved liver phospholipids fatty acid composition and parameters of oxidative stress in male wistar rats. J Anim Physiol Anim Nutr. doi:10.1111/j.1439-0396.2011.01216.x
Abeywardena MY, Patten GS (2011) Role of ω3 long-chain polyunsaturated fatty acids in reducing cardio-metabolic risk factors. Endocr Metab Immune Disord Drug Targets 11(3):232–246. ISSN: 2212–3873
Westphal S, Orth M, Ambrosch A, Osmundsen K, Luley C (2000) Postprandial chylomicrons and VLDLs in severe hypertriacylglycerolemia are lowered more effectively than are chylomicron remnants after treatment with n-3 fatty acids. Am J Clin Nutr 71(4):914–920
Roche HM, Gibney MJ (2000) Effect of long-chain n-3 polyunsaturated fatty acids on fasting and postprandial triacylglycerol metabolism. Am J Clin Nutr 71(1 Suppl):232S–237S
Fox JC, Hay RV (1992) Eicosapentaenoic acid inhibits cell growth and triacylglycerol secretion in McA-RH7777 rat hepatoma cultures. Biochem J 286(Pt 1):305–312
Benner KG, Sasaki A, Gowen DR, Weaver A, Connor WE (1990) The differential effect of eicosapentaenoic acid and oleic acid on lipid synthesis and VLDL secretion in rabbit hepatocytes. Lipids 25(9):534–540
Chen HW, Lii CK, Ko JJ, Wang ST, Hsu JD (1996) Regulatory effects of dietary n-3 and n-6 lipids on plasma and hepatic lipid levels, liver cell number and microsomal protein content in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids 55(5):329–335
Willumsen N, Skorve J, Hexeberg S, Rustan AC, Berge RK (1993) The hypotriglyceridemic effect of eicosapentaenoic acid in rats is reflected in increased mitochondrial fatty acid oxidation followed by diminished lipogenesis. Lipids 28(8):683–690
Skulas-Ray AC, Kris-Etherton PM, Harris WS, vanden Heuvel JP, Wagner PR, West SG (2011) Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 93(2):243–252
Oya J, Nakagami T, Sasaki S, Jimba S, Murakami K, Kasahara T et al (2010) Intake of n-3 polyunsaturated fatty acids and non-alcoholic fatty liver disease: a cross-sectional study in Japanese men and women. Eur J Clin Nutr 64(10):1179–1185
Hodge J, Sanders K, Sinclair AJ (1993) Differential utilization of eicosapentaenoic acid and docosahexaenoic acid in human plasma. Lipids 28(6):525–531
Yep YL, Li D, Mann NJ, Bode O, Sinclair AJ (2002) Bread enriched with microencapsulated tuna oil increases plasma docosahexaenoic acid and total omega-3 fatty acids in humans. Asia Pac J Clin Nutr 11(4):285–291
Conquer JA, Cheryk LA, Chan E, Gentry PA, Holub BJ (1999) Effect of supplementation with dietary seal oil on selected cardiovascular risk factors and hemostatic variables in healthy male subjects. Thromb Res 96(3):239–250
Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD et al (2000) Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr 71(5):1085–1094
Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY (2006) Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem [Randomized Controlled Trial] 52(12):2265–2272
Zuijdgeest-van Leeuwen SD, Dagnelie PC, Rietveld T, van den Berg JW, Wilson JH (1999) Incorporation and washout of orally administered n-3 fatty acid ethyl esters in different plasma lipid fractions. Br J Nutr 82(6):481–488
Christensen E, Woldseth B, Hagve TA, Poll-The BT, Wanders RJ, Sprecher H et al (1993) Peroxisomal beta-oxidation of polyunsaturated long chain fatty acids in human fibroblasts. The polyunsaturated and the saturated long chain fatty acids are retroconverted by the same acyl-CoA oxidase. Scand J Clin Lab Invest Suppl 215:61–74
Stoffel W, Eker A, Assad H, Sprecher H (1970) Enzymatic studies on the mechanism of the retroconversion of C22-polyenoic fatty acids to their C20-homologues. Hoppe Seylers Z Physiol Chem 351(12):1545–1554
Achard F, Benistant C, Lagarde M (1995) Interconversions and distinct metabolic fate of eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in bovine aortic endothelial cells. Biochim Biophys Acta 1255(3):260–266
Rosenthal MD, Garcia MC, Jones MR, Sprecher H (1991) Retroconversion and delta 4 desaturation of docosatetraenoate (22:4(n-6)) and docosapentaenoate (22:5(n-3)) by human cells in culture. Biochim Biophys Acta 1083(1):29–36
Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38(10):2012–2022
Krul ES, Lemke SL, Mukherjea R, Taylor ML, Goldstein DA, Su H et al (2012) Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot Essent Fatty Acids 86(1–2):51–59
Meyer BJ, Lane AE, Mann NJ (2009) Comparison of seal oil to tuna oil on plasma lipid levels and blood pressure in hypertriglyceridaemic subjects. Lipids 44(9):827–835
Brown AJ, Pang E, Roberts DC (1991) Erythrocyte eicosapentaenoic acid versus docosahexaenoic acid as a marker for fish and fish oil consumption. Prostaglandins Leukot Essent Fatty Acids 44(2):103–106
Brown AJ, Pang E, Roberts DC (1991) Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of n-3 polyunsaturated fatty acids: study design implications. Am J Clin Nutr 54(4):668–673
Cartwright IJ, Pockley AG, Galloway JH, Greaves M, Preston FE (1985) The effects of dietary omega-3 polyunsaturated fatty acids on erythrocyte membrane phospholipids, erythrocyte deformability and blood viscosity in healthy volunteers. Atherosclerosis 55(3):267–281
Acknowledgments
Research support from Meat & Livestock Australia for financial support, Equateq Ltd (UK) for the generous provision of the pure supplements and Deakin University Strategic Research Centre for Molecular Medicine for financial support is gratefully acknowledged. EM, AL, DCS and AJS planned and designed the study; EM and AL recruited the participants and collected samples and dietary data; GK, EM and GT conducted the plasma analyses; GK, SPL and GT conducted the RBC analyses; GK conducted the statistical analysis; GK, AJS and DCS wrote the manuscript; GK, AJS, DCS, KL and HSW made significant contributions to the discussion.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eliza Miller and Gunveen Kaur contributed equally to this work.
Rights and permissions
About this article
Cite this article
Miller, E., Kaur, G., Larsen, A. et al. A short-term n-3 DPA supplementation study in humans. Eur J Nutr 52, 895–904 (2013). https://doi.org/10.1007/s00394-012-0396-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0396-3