Abstract
Background and aims
The adenomatous polyposis coli (APC) protein plays a crucial role in the regulation of β-catenin, which is linked to the cell adhesion molecule E-cadherin. Furthermore, β-catenin and cyclooxygenase-2 (COX-2) are both involved in the activation of nuclear transcription factors inducing cell proliferation. Germline mutations in the APC gene are the cause of familial adenomatous polyposis (FAP). To characterise the expression pattern of these proteins in FAP in comparison with sporadic adenomas, we studied 18 FAP-associated adenomas, 16 sporadic adenomas and seven normal colonic controls.
Methods
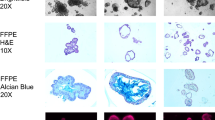
E-cadherin, β-catenin, COX-2 expression and the proliferative index (Ki67) were assessed by immunohistochemistry (index of expressing cells / total number of cells) in adenomatous mucosa, adjacent non-neoplastic tissue and normal colonic controls.
Results
E-cadherin expression was significantly and homogeneously reduced in FAP adenomas (24%; 95%CI 16–32; sporadic adenomas 61%; 38–84; normal controls 98%; 96–100). Membraneous β-catenin expression was significantly reduced in both FAP (30%; 11–49) and sporadic (42%; 19–65) adenomas (normal controls 96%; 88–104), whereas marked nuclear staining occurred in sporadic, but not in FAP adenomas. Stromal COX-2 expression and the proliferative index were increased only in sporadic adenomas (sporadic adenomas: COX-2 12%; 7–17, Ki67 24%; 15–33, FAP adenomas: COX-2 8%; 5–11, Ki67 5%; 2–9, normal controls: COX-2 4%; 2–7, Ki67 6%; 1–11).
Conclusion
Proteins involved in cell adhesion and cell proliferation, especially E-cadherin, are expressed differently in FAP and sporadic adenoma, pointing to possible differences in the molecular pathways to adenoma.





Similar content being viewed by others
References
Kinzler KW, Vogelstein B (1996) Lessons from hereditary colorectal cancer. Cell 87:159–170
Knudsen KA, Soler AP, Johnson KR, Wheelock MJ (1995) Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J Cell Biol 130:67–77
Behrens J, Mareel MM, Van Roy FM, Birchmeier W (1989) Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol 108:2435–2447
Bienz M, Clevers H (2000) Linking colorectal cancer to Wnt signaling. Cell 103:311–320
Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, et al (1999) The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A 96:5522–5527
Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426
He TC, Chan TA, Vogelstein B, Kinzler KW (1999) PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell 99:335–345
Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al (1997) Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 275:1787–1790
Jass JR, Whitehall VLJ, Young J, Leggett BA (2002) Emerging concepts in colorectal neoplasia. Gastroenterology 123:862–876
Van Aken J, Cuvelier CA, De Wever N, Roels J, Gao Y, Mareel MM (1993) Immunohistochemical analysis of E-cadherin expression in human colorectal tumours. Pathol Res Pract 189:975–978
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Mandl M, Paffenholz R, Friedl W, Caspari R, Sengteller M, Propping P (1994) Frequency of common and novel inactivating APC mutations in 202 families with familial adenomatous polyposis. Hum Mol Genet 3:181–184
Bala S, Kraus C, Wijnen J, Meera Khan P, Ballhausen WG (1996) Multiple products in the protein truncation test due to alternative splicing in the adenomatous polyposis coli (APC) gene. Hum Genet 98:528–533
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580
Gagliardi G, Kandemir O, Liu D, Guida M, Benvestito S, Ruers TG, et al (1995) Changes in E-cadherin immunoreactivity in the adenoma-carcinoma sequence of the large bowel. Virchows Arch 426:149–154
Valizadeh A, Karayiannakis AJ, el-Hariry I, Kmiot W, Pignatelli M (1997) Expression of E-cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120) in colorectal polyps. Am J Pathol 150:1977–1984
Ashida K, Terada T, Kitamura Y, Kaibara N (1998) Expression of E-cadherin, alpha-catenin, beta-catenin, and CD44 (standard and variant isoforms) in human cholangiocarcinoma: an immunohistochemical study. Hepatology 27:974–982
Jorgensen OD, Kronborg O, Fenger C (1993) The Funen Adenoma Follow-Up Study. Characteristics of patients and initial adenomas in relation to severe dysplasia. Scand J Gastroenterol 28:239–243
Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S (1996) Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res 56:2213–2217
El-Bahrawy MA, Talbot IC, Poulsom R, Jeffery R, Alison MR (2002) The expression of E-cadherin and catenins in colorectal tumours from familial adenomatous polyposis patients. J Pathol 198:69–76
Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, et al (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120:757–766
Kobayashi M, Honma T, Matsuda Y, Suzuki Y, Narisawa R, Ajioka Y, et al (2000) Nuclear translocation of beta-catenin in colorectal cancer. Br J Cancer 82:1689–1693
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R (1996) Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev 59:3–10
Herter P, Kuhnen C, Muller KM, Wittinghofer A, Muller O (1999) Intracellular distribution of beta-catenin in colorectal adenomas, carcinomas and Peutz-Jeghers polyps. J Cancer Res Clin Oncol 125:297–304
Mahmoud NN, Boolbol SK, Dannenberg AJ, Mestre JR, Bilinski RT, Martucci C, et al (1998) The sulfide metabolite of sulindac prevents tumors and restores enterocyte apoptosis in a murine model of familial adenomatous polyposis. Carcinogenesis 19:87–91
Weiss H, Jacobasch KH, Haensch W, Streller B, Hieke B (1997) Significance of apoptosis in the process of tumorigenesis in colorectal mucosa and adenomas in FAP patients. Anal Cell Pathol 14:61–73
Peifer M (1997) Beta-catenin as oncogene: the smoking gun. Science 275:1752–1753
Muller-Decker K, Albert C, Lukanov T, Winde G, Marks F, Furstenberger G (1999) Cellular localization of cyclo-oxygenase isozymes in Crohn’s disease and colorectal cancer. Int J Colorectal Dis 14:212–218
Bamba H, Ota S, Kato A, Adachi A, Itoyama S, Matsuzaki F (1999) High expression of cyclooxygenase-2 in macrophages of human colonic adenoma. Int J Cancer 83:470–475
Acknowledgements
We thank Mrs. Ursula Becker for excellent technical assistance and Dr. Schäfer and Dr. Schulte-Witte for their support. This work was supported by the Ludwig-Demling-Stipendium (Olympus optical, Hamburg, Germany), the BONFOR-programme of the University of Bonn, Germany, and the Deutsche Krebshilfe (German cancer aid, Bonn, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jungck, M., Grünhage, F., Spengler, U. et al. E-cadherin expression is homogeneously reduced in adenoma from patients with familial adenomatous polyposis: an immunohistochemical study of E-cadherin, β-catenin and cyclooxygenase-2 expression. Int J Colorectal Dis 19, 438–445 (2004). https://doi.org/10.1007/s00384-003-0575-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-003-0575-z