Abstract
Background
Intestinal adaptation in short bowel syndrome (SBS) consists of increased epithelial cells (ECs) proliferation as well as apoptosis. Angiotensin-converting enzyme (ACE) has been shown to regulate ECs apoptosis. In this study, we investigated the effect of ACE inhibition on intestinal adaptation after small bowel resection (SBR) in a rat model.
Methods
Sprague–Dawley rats were used and were divided into four groups: (1) Sham group received an ileum transection (n = 6); (2) Sham + ACE-I group received an ileum transaction and lavage with ACE inhibitor (ACE-I, enalaprilat, 2 mg/kg/day) (n = 6); (3) SBS group received a 70 % mid-intestinal resection (n = 6); (4) SBS + ACE-I group received a 70 % mid-intestinal resection and lavage with enalaprilat (2 mg/kg/day) (n = 6). Sampling was done 10 days after surgery. ECs apoptosis was studied by TUNEL staining. ACE, angiotensin II (ANGII) receptor type 1 (AT1R) and receptor type 2 (AT2R) expressions were detected with RT-PCR and immunofluorescent confocal microscopy.
Results
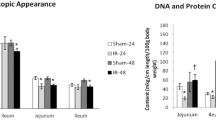
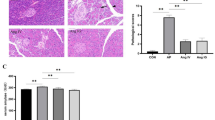
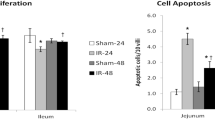
SBR leads to significant intestinal hypertrophy. The addition of ACE-I to SBS rat resulted in a significant decline in ECs apoptosis. ACE mRNA expression was significantly elevated after SBS creation (0.24 ± 0.07 vs. 0.42 ± 0.11), and ACE-I administration further increased mucosal ACE mRNA expression (0.54 ± 0.12). Interestingly, AT1R mRNA expression showed a significant decline in the SBS group compared to Sham levels, and ACE-I administration increased AT1R mRNA expression to Sham levels. No significant difference in AT2R mRNA expression was found between Sham and SBS group.
Conclusion
These results offer further insight into the role of ACE on intestinal mucosal remolding after massive bowel resection. ACE-I may be beneficial to SBS patients via a reduction of the apoptotic rate, thus facilitating the degree of adaptation.







Similar content being viewed by others
References
Westergaard H (2002) Short bowel syndrome. Semin Gastrointest Dis 13:210–220
Vanderhoof JA, Langnas AN, Pinch LW et al (1992) Short bowel syndrome. J Pediatr Gastroenterol Nutr 14:359–370
Stern LE, Huang F, Kemp CJ et al (2000) Bax is required for increased enterocyte apoptosis after massive small bowel resection. Surgery 128:165
Wildhaber BE, Yang H, Coran AG et al (2003) Gene alteration of intestinal intraepithelial lymphocytes in response to massive small bowel resection. Pediatr Surg Int 19:310
Welters CF, Dejong CH, Deutz NE et al (2002) Intestinal adaptation in short bowel syndrome. ANZ J Surg 72:229
Wildhaber BE, Yang H, Haxhija EQ et al (2005) Intestinal intraepithelial lymphocyte derived angiotensin converting enzyme modulates epithelial cell apoptosis. Apoptosis 10:1305–1315
Danilov SM, Faerman AI, Printseva O et al (1987) Immunohistochemical study of angiotensin converting enzyme in human tissues using monoclonal antibodies. Histochemistry 87:487–490
Wang R, Zagariya A, Ibarra-Sunga O et al (1999) Angiotensin II induces apoptosis in human and rat alveolar epithelial cells. Am J Physiol 276:L885–L889
Yu G, Liang X, Xie X et al (2001) Diverse effects of chronic treatment with losartan, fosinopril, and amlodipine on apoptosis, angiotensin II in the left ventricle of hypertensive rats. Int J Cardiol 81:123–129 (Discussion 129–130)
Watanabe M, Kawaguchi H, Onozuka H et al (1998) Chronic effects of enalapril and amlodipine on cardiac remodeling in cardiomyopathic hamster hearts. J Cardiovasc Pharmacol 32:248
Yang H, Larsson J, Permert J et al (2000) Bolus ornithine and arginine ketoglutarate supplementation in distal intestine after 65 % resection in rats. Nutr Res 20(12):1807–1816
Grossmann J, Maxson J, Whitacre C et al (1998) New isolation technique to study apoptosis in human intestinal epithelial cells. Am J Pathol 153:53–62
Yang H, Spencer AU, Teitelbaum DH et al (2005) Interleukin-7 administration alters intestinal intraepithelial lymphocyte phenotype and function in vivo. Cytokine 31:419–428
Haxhija EQ, Yang H, Spencer AU et al (2006) Influence of the site of small bowel resection on intestinal epithelial cell apoptosis. Pediatr Surg Int 22:37–42
Helmrath M, Fong J, Dekaney C et al (2007) Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol 292:G215–G222
Odaka C, Mizuochi T (2000) Angiotensin-converting enzyme inhibitor captopril prevents activation-induced apoptosis by interfering with T cell activation signals. Clin Exp Immunol 121:515–522
Wang R, Ramos C, Joshi I et al (1999) Human lung myofibroblast-derived inducers of alveolar epithelial apoptosis identified as angiotensin peptides. Am J Physiol 277:L1158–L1164
Coopersmith CM, Gordon JI (1999) Bcl-2 inhibits ischemia–reperfusion-induced apoptosis in the intestinal epithelium of transgenic mice. Am J Physiol 276:G677–G686
Yang H, Fan Y, Finaly R et al (2003) Alteration of intestinal intraepithelial lymphocytes after massive small bowel resection. J Surg Res 110:276–286
Knott AW, Juno RJ, Jarboe MD et al (2003) EGF receptor signaling affects bcl-2 family gene expression and apoptosis after massive small bowel resection. J Pediatr Surg 38:875–880
Yang H, Antony PA, Wildhaber BE et al (2004) Intestinal intraepithelial lymphocyte gamma-delta T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol 172:4151–4158
Tang Y, Swartz-Basile D, Swietlicki E et al (2004) Bax is required for resection-induced changes in apoptosis, proliferation, and members of the extrinsic cell death pathways. Gastroenterology 126:220–225
Haxhija EQ, Hua Y et al (2008) Modulation of mouse intestinal epithelial cell turnover in the absence of angiotensin converting enzyme. Am J Physiol Gastrointest Liver Physiol 295:G88–G98
Wang R, Ibarra-Sunga O, Verlinski L et al (2000) Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol 279:L143–L151
Schiffrin EL (2002) Vascular and cardiac benefits of angiotensin receptor blockers. Am J Med 113:409–418
Siragy HM (2000) AT(1) and AT(2) receptors in the kidney: role in disease and treatment. Am J Kidney Dis 36:S4–S9
Suzuki J, Iwai M, Nakagami H et al (2002) Role of angiotensin II-regulated apoptosis through distinct AT1 and AT2 receptors in neointimal formation. Circulation 106:847–852
Li X, Rayford H, Uhal BD (2003) Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol 163:2523–2530
Schiffrin E (1992) Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture. Hypertension 19:1–9
Acknowledgments
This research was supported by the National Natural Science Foundation of China (NSFC 30973113, NSFC 30872465, NSFC 81020108023), the Scientific Research Foundation of The Third Military Medical University for the Returned Overseas Chinese Scholars and Chongqing Science and Technology Commission (CSTC2008BA5006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Xiao, W., Sun, L. et al. Inhibition of ACE activity contributes to the intestinal structural compensation in a massive intestinal resection rat model. Pediatr Surg Int 28, 533–541 (2012). https://doi.org/10.1007/s00383-012-3075-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-012-3075-9