Abstract
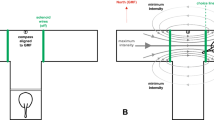
Orientation by magnetic cues appears to be adaptive during animal migrations. Whereas the magnetic orientation in birds, mammals, and urodele amphibians is being investigated intensively, the data about anurans are still scarce. This study tests whether marsh frogs could determine migratory direction between the breeding pond and the wintering site by magnetic cues in the laboratory. Adult frogs (N = 32) were individually tested in the T-maze 127 cm long inside the three-axis Helmholtz coil system (diameter 3 m). The arms of the maze were positioned parallel to the natural migratory route of this population when measured in accordance with magnetic field. The frogs were tested under two-motivational conditions mediated by temperature/light regime: the breeding migratory state and the wintering state. The frogs’ choice in a T-maze was evident only when analyzed in accordance with the direction of the magnetic field: they moved along the migratory route to the breeding pond and followed the reversion of the horizontal component of the magnetic field. This preference has been detected in both sexes only in the breeding migratory state. This suggests that adult ranid frogs can obtain directional information from the Earth’s magnetic field as was shown earlier in urodeles and anuran larvae.

Similar content being viewed by others
References
Adler K (1980) Individuality in the use of orientation cues by green frogs. Anim Behav 28:413–425. doi:10.1016/S0003-3472(80)80050-9
Adler K (1982) Sensory aspects of amphibian navigation and compass orientation. Vertebr Hung 21:7–18
Alexandrovskaya T, Bikov D (1979) The distribution of green frogs in the European part of the USSR. In: Proceedings of VII zoogeographic conference, Moscow, pp 279–281 (in Russian)
Deutschlander ME, Phillips JB, Borland SC (1999) The case for light-dependent magnetic orientation in animals. J Exp Biol 202:891–908
Diego-Rasilla FJ (2003) Homing ability and sensitivity to the geomagnetic field in the alpine newt, Triturus alpestris. Ethol Ecol Evol 15:251–259. doi:10.1080/08927014.2003.9522670
Diego-Rasilla J, Luengo R (2002) Celestial orientation in the marbled newt (Triturus marmoratus). J Ethol 20:137–141. doi:10.1007/s10164-002-0066-7
Diego-Rasilla FJ, Luengo RM, Phillips JB (2010) Light-dependent magnetic compass in Iberian green frog tadpoles. Naturwissenschaften 97:1077–1088. doi:10.1007/s00114-010-0730-7
Diego-Rasilla FJ, Luengo RM, Phillips JB (2013) Use of a light-dependent magnetic compass for Y-axis orientation in European common frog (Rana temporaria) tadpoles. J Comp Physiol A 199:619–628. doi:10.1007/s00359-013-0811-0
Eilam D (2003) Open-field behavior withstands drastic changes in arena size. Behav Brain Res 142:53–62. doi:10.1016/S0166-4328(02)00382-0
Ferguson DE, McKeown JP, Bosarge OS, Landreth HF (1968) Sun-compass orientation of bullfrogs. Copeia 1968:230. doi:10.2307/1441746
Freake MJ, Phillips JB (2005) Light-dependent shift in bullfrog tadpole magnetic compass orientation: evidence for a common magnetoreception mechanism in anuran and urodele amphibians. Ethology 111:241–254. doi:10.1111/j.1439-0310.2004.01067.x
Freake MJ, Borland SC, Phillips JB (2002) Use of a magnetic compass for Y-axis orientation in larval bullfrogs, Rana catesbeiana. Copeia 2002:466–471. doi:10.1643/0045-8511(2002)002[0466:UOAMCF]2.0.CO;2
Grant D, Anderson O, Twitty V (1968) Homing orientation by olfaction in newts (Taricha rivularis). Science 160:1354–1356. doi:10.1126/science.160.3834.1354
Grubb JC (1973a) Olfactory orientation in breeding mexican toads, Bufo valliceps. Copeia 1973:490. doi:10.2307/1443114
Grubb JC (1973b) Olfactory orientation in Bufo woodhousei fowleri, Pseudacris clarki and Pseudacris streckeri. Anim Behav 21:726–732. doi:10.1016/S0003-3472(73)80098-3
Kim JW, Im WB, Choi HH et al (1998) Seasonal fluctuations in pituitary gland and plasma levels of gonadotropic hormones in Rana. Gen Comp Endocrinol 109:13–23. doi:10.1006/gcen.1997.6992
Kirschvink JL, Jones DS, MacFadden BJ (eds) (1985) Magnetite biomineralization and magnetoreception in organisms, 1st edn. Springer, Boston
Kishkinev D, Chernetsov N, Pakhomov A et al (2015) Eurasian reed warblers compensate for virtual magnetic displacement. Curr Biol 25:R822–R824. doi:10.1016/j.cub.2015.08.012
Kuzmin SL (2013) The Amphibians of the Former Soviet Union, 2nd edn Revised. Pensoft Publishers, Sofia – Moscow
Lada GA, Borkin LJ, Vinogradov AE (1995) Distribution, population systems and reproductive behavior of green frogs (hybridogenetic Rana esculenta complex) in the Central Chernozem Territory of Russia. Russ J Herpetol 2:46–57
Landler L, Gollmann G (2011) Magnetic orientation of the common toad: establishing an arena approach for adult anurans. Front Zool 8:6. doi:10.1186/1742-9994-8-6
Lebedev IV, Pleskacheva MG, Anokhin KV (2012) C57BL/6 mice open field behaviour qualitatively depends on arena size. Zhurnal Vyss Nervn deiatelnosti Im I P Pavlov 62:485–496
Lehner PN (1996) Handbook of ethological methods, 2nd edn. Cambridge University Press, Cambridge
Lohmann KJ, Lohmann CMF (1993) A light-independent magnetic compass in the leatherback sea turtle. Biol Bull 185:149. doi:10.2307/1542138
Marhold S, Wiltschko W, Burda H (1997) A magnetic polarity compass for direction finding in a subterranean mammal. Naturwissenschaften 84:421–423. doi:10.1007/s001140050422
Marty P, Angélibert S, Giani N, Joly P (2005) Directionality of pre- and post-breeding migrations of a marbled newt population (Triturus marmoratus): implications for buffer zone management. Aquat Conserv Mar Freshw Ecosyst 15:215–225. doi:10.1002/aqc.672
Merrell DJ (1970) Migration and gene dispersal in Rana pipiens. Integr Comp Biol 10:47–52. doi:10.1093/icb/10.1.47
Mouritsen H (2013) The magnetic senses. In: Galizia CG, Lledo P-M (eds) Neurosciences—from molecule to behavior: a university textbook. Springer, Berlin, pp 427–443
Mouritsen H (2015) Magnetoreception in birds and its use for long-distance migration. In: Scanes CG (ed) Sturkie’s avian physiology, 6th edn. Academic Press, New York, pp 113–133
Muheim R, Boström J, Åkesson S, Liedvogel M (2014) Sensory mechanisms of animal orientation and navigation. In: Hansson L-A, Åkesson S (eds) Animal movement across scales. Oxford University Press, Oxford, pp 179–194
Nakazawa H, Ishii S (2000) Changes in the oscillatory electric potential on the olfactory epithelium and in reproductive hormone levels during the breeding season in the toad (Bufo japonicus). Zool Sci 592:585–592. doi:10.2108/zsj.17.585
Nakazawa H, Kaji S, Ishii S (2000) Oscillatory electric potential on the olfactory epithelium observed during the breeding migration period in the japanese toad, Bufo japonicus. Zool Sci 17:293–300. doi:10.2108/jsz.17.293
Ogurtsov SV (2004) Olfactory orientation in anuran amphibians. Russ J Herpetol 11:35–40
Ogurtsov SV (2005) Basis of native pond fidelity in anuran amphibians: the case of chemical learning. Russ J Herpetol 12:198–200
Oldham RS (1966) Spring movements in the American toad, Bufo Americanus. Can J Zool 44:63–100. doi:10.1139/z66-006
Phillips J (1986) Two magnetoreception pathways in a migratory salamander. Science 233:765–767. doi:10.1126/science.3738508
Phillips JB (1987) Laboratory studies of homing orientation in the eastern red-spotted newt, Notophthalmus viridescens. J Exp Biol 131:215–229
Phillips JB, Borland SC (1992) Behavioural evidence for use of a light-dependent magneto reception mechanism by a vertebrate. Nature 359:142–144. doi:10.1038/359142a0
Phillips JB, Deutschlander ME, Freake MJ, Borland SC (2001) The role of extraocular photoreceptors in newt magnetic compass orientation: parallels between light-dependent magneto reception and polarized light detection in vertebrates. J Exp Biol 204:2543–2552
Phillips JB, Jorge PE, Muheim R (2010) Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanisms. J R Soc Interface 7(Suppl 2):S241–S256. doi:10.1098/rsif.2009.0459.focus
Polzonetti-Magni AM, Mosconi G, Carnevali O et al (1998) Gonadotropins and reproductive function in the anuran amphibian, Rana esculenta. Biol Reprod 58:88–93. doi:10.1095/biolreprod58.1.88
Rastogi RK, Iela L, Delrio G et al (1978) Environmental influence on testicular activity in the green frog, Rana esculenta. J Exp Zool 206:49–63. doi:10.1002/jez.1402060106
Reshetnikov AN (1996) Hygrotactic and olfactory orientation in juvenile common toads (Bufo bufo) during the postmetamorphic period. Adv Amphib Res Former Sov Union 1:181–190
Rozhok A (2008) Orientation and navigation in vertebrates. Springer, Berlin
Schlegel PA (2007) Spontaneous preferences for magnetic compass direction in the American red-spotted newt, Notophthalmus viridescens (Salamandridae, Urodela). J Ethol 25:177–184. doi:10.1007/s10164-006-0016-x
Shakhparonov VV (2012) The influence of repeated experiments on the behavior of the marsh frog (Rana ridibunda) in field experiments of spatial orientation. Zool Zurnal 91:1340–1350
Shakhparonov VV, Ogurtsov SV (2008) Seasonal and geographical variability of orientation behavior in the marsh frog (Rana ridibunda) in search of its own water body. Zool Zurnal 87:1062–1076
Shoop CR (1965) Orientation of Ambystoma maculatum: movements to and from breeding ponds. Science 149:558–559. doi:10.1126/science.149.3683.558
Sinsch U (1987) Orientation behaviour of toads (Bufo bufo) displaced from the breeding site. J Comp Physiol A 161:715–727
Sinsch U (1988a) El sapo andino Bufo spinulosus: análisis preliminar de su orientación hacia sus lugares de reproducción. Bol Lima 57:83–92
Sinsch U (1988b) Seasonal changes in the migratory behaviour of the toad Bufo bufo: direction and magnitude of movements. Oecologia 76:390–398. doi:10.1007/bf00377034
Sinsch U (1990a) Migration and orientation in anuran amphibians. Ethol Ecol Evol 2:65–79. doi:10.1080/08927014.1990.9525494
Sinsch U (1990b) The orientation behaviour of three toad species (genus Bufo) displaced from the breeding site. Fortschr Zool 38:75–83
Sinsch U (1992) Amphibians. In: Papi F (ed) Homing animal. Chapman & Hall, London, pp 213–233
Sinsch U, Kirst C (2015) Homeward orientation of displaced newts (Triturus cristatus, Lissotriton vulgaris) is restricted to the range of routine movements. Ethol Ecol Evol. doi:10.1080/03949370.2015.1059893
Taylor DH, Adler K (1973) Spatial orientation by salamanders using plane-polarized light. Science 181:285–287. doi:10.1126/science.181.4096.285
Voituron Y (2005) Freezing tolerance of the European water frogs: the good, the bad, and the ugly. AJP Regul Integr Comp Physiol 288:R1563–R1570. doi:10.1152/ajpregu.00711.2004
Voituron Y, Eugene M, Barré H (2003) Survival and metabolic responses to freezing by the water frog (Rana ridibunda). J Exp Zool Part A Comp Exp Biol 299A:118–126. doi:10.1002/jez.a.10285
Wells KD (2015) The ecology and behavior of amphibians. The University of Chicago Press, Chicago
Wiltschko W, Wiltschko R (1972) Magnetic compass of European robins. Science 176:62–64. doi:10.1126/science.176.4030.62
Wiltschko W, Wiltschko R (2005) Magnetic orientation and magneto reception in birds and other animals. J Comp Physiol A 191:675–693. doi:10.1007/s00359-005-0627-7
Acknowledgements
We would like to thank A.V. Spassky and V.M. Lebedev (Skobeltsyn Institute of Nuclear Physics of MSU) for the technical assistance while working with Helmholtz coil system. We are also thankful to A.P. Golovlev, A.S. Dubrovskaya, and E.E. Gritsyshina for their help during the experiments. Magnetometer GI MTS-1 was kindly provided by N.S. Chernetsov from Zoological Institute RAS. The work of V.V. Shakhparonov was financed by Grant mol_a 14-04-32243 from the Russian Foundation for Basic Research. Bartington Mag658 Digital Three-Axis Fluxgate Magnetometer was obtained with the support of the Russian Science Foundation, Grant No. 14-50-00029 “Scientific Basis of the National Biobank—Depository of the Living Systems”. The Helmholtz coil system was kindly provided in accordance with the Program of Development of Lomonosov Moscow State University. We are grateful to two anonymous reviewers for very useful comments that greatly improved the presentation of the material and to Maria Wilding for her kind assistance in the editing of the English language of the manuscript. All applicable international, national, and institutional guidelines for the care and use of animals were followed, including “Guidelines for accommodation and care of animals” of the “European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes” (ETS No. 123) and “Guidelines for the treatment of animals in behavioral research and teaching” produced by the Association for the Study of Animal Behavior and the Animal Behavior Society (Animal Behavior (2012) 83:301–309). This study was conducted in agreement with the legislation of the Russian Federation and with the requirements of the Committee for Bio Ethics of Lomonosov Moscow State University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shakhparonov, V.V., Ogurtsov, S.V. Marsh frogs, Pelophylax ridibundus, determine migratory direction by magnetic field. J Comp Physiol A 203, 35–43 (2017). https://doi.org/10.1007/s00359-016-1132-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-016-1132-x