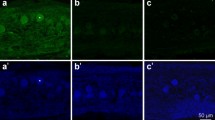
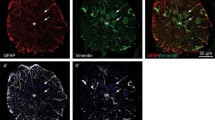
Abstract
In contrast to mammals, teleost fish exhibit an enormous potential to regenerate adult spinal cord tissue after injury. However, the mechanisms mediating this ability are largely unknown. Here, we analyzed the major processes underlying structural and functional regeneration after amputation of the caudal portion of the spinal cord in Apteronotus leptorhynchus, a weakly electric teleost. After a transient wave of apoptotic cell death, cell proliferation started to increase 5 days after the lesion and persisted at high levels for at least 50 days. New cells differentiated into neurons, glia, and ependymal cells. Retrograde tract tracing revealed axonal re-growth and innervation of the regenerate. Functional regeneration was demonstrated by recovery of the amplitude of the electric organ discharge, a behavior generated by spinal motoneurons. Computer simulations indicated that the observed rates of apoptotic cell death and cell proliferation can adequately explain the re-growth of the spinal cord.









Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BrdU:
-
5-bromo-2′-deoxyuridine
- BSA:
-
Bovine serum albumine
- CNS:
-
Central nervous system
- DAPI:
-
4′,6-diamidino-2-phenylindoledihydrochloride
- EOD:
-
Electric organ discharge
- GFAP:
-
Glial fibrillary acidic protein
- PB:
-
Phosphate buffer
- PBS:
-
Phosphate-buffered saline
- SCI:
-
Spinal cord injury
- SD:
-
Standard deviation
- SSC:
-
Saline-sodium citrate (buffer)
- TBS:
-
Tris-buffered saline
References
Alvarez-Buylla A, García-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2:287–293
Bass AH (1986) Evolution of a vertebrate communication and orientation organ. In: Bullock TH, Heiligenberg W (eds) Electroreception. Wiley, New York, pp 13–70
Beattie MS (2004) Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med 10:580–583
Beattie MS, Hermann GE, Rogers RC, Bresnahan JC (2002) Cell death in models of spinal cord injury. Prog Brain Res 137:37–47
Becker CG, Becker T (2007) Zebrafish as a model system for successful spinal cord regeneration. In: Becker CG, Becker T (eds) Model organisms in spinal cord regeneration. Wiley-VCH, Weinheim, pp 289–319
Becker CG, Lieberoth BC, Morellini F, Feldner J, Becker T, Schachner M (2004) L1.1 is involved in spinal cord regeneration in adult zebrafish. J Neurosci 24:7837–7842
Bennett MVL (1971) Electric organs. In: Randall DJ (ed) Fish physiology: sensory systems and electric organs. Academic Press, New York, pp 347–489
Benraiss A, Arsanto JP, Coulon J, Thouveny Y (1999) Neurogenesis during caudal spinal cord regeneration in adult newts. Dev Genes Evol 209:363–369
Bursch W, Paffe S, Putz B, Barthel G, Schulte-Hermann R (1990) Determination of the length of the histological stages of apoptosis in normal liver and in altered hepatic foci of rats. Carcinogenesis 11:847–853
Busch SA, Silver J (2007) The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol 17:120–127
Chernoff EAG, Sato K, Corn A, Karcavich RE (2002) Spinal cord regeneration: intrinsic properties and emerging mechanisms. Semin Cell Dev Biol 13:361–368
Chevallier S, Landry M, Nagy F, Cabelguen JM (2004) Recovery of bimodal locomotion in the spinal-transected salamander, Pleurodeles waltlii. Eur J Neurosci 20:1995–2007
Clint SC, Zupanc GKH (2001) Neuronal regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: guidance of migrating young cells by radial glia. Dev Brain Res 130:15–23
Clint SC, Zupanc GKH (2002) Up-regulation of vimentin expression during regeneration in the adult fish brain. NeuroReport 13:317–320
Doyle LMF, Stafford PP, Roberts BL (2001) Recovery of locomotion correlated with axonal regeneration after a complete spinal transection in the eel. Neuroscience 107:169–179
Echeverri K, Tanaka EM (2002) Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science 298:1993–1996
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Endo T, Yoshino J, Kado K, Tochinai S (2007) Brain regeneration in anuran amphibians. Dev Growth Differ 49:121–129
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25:1439–1451
Ferretti P, Zhang F, O’Neill P (2003) Changes in spinal cord regenerative ability through phylogenesis and development: lessons to be learnt. Dev Dyn 226:245–256
Filbin MT (2006) Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B 361:1565–1574
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294–301
Horky LL, Galimi F, Gage FH, Horner PJ (2006) Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol 498:525–538
Huard JMT, Schwob JE (1995) Cell cycle of globose basal cells in rat olfactory epithelium. Dev Dyn 203:17–26
Johansson BB (2007) Regeneration and plasticity in the brain and spinal cord. J Cereb Blood Flow Metab 27:1417–1430
Klussmann S, Martin-Villalba A (2005) Molecular targets in spinal cord injury. J Mol Med 83:657–671
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR (2004) Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 4:451–464
Lawson SJ, Lowrie MB (1998) The role of apoptosis and excitotoxicity in the death of spinal motoneurons and interneurons after neonatal nerve injury. Neuroscience 87:337–348
Lim PAC, Tow AM (2007) Recovery and regeneration after spinal cord injury: a review and summary of recent literature. Ann Acad Med Singapore 36:49–57
Maier IC, Schwab ME (2006) Sprouting, regeneration and circuit formation in the injured spinal cord: factors and activity. Philos Trans R Soc Lond B 361:1611–1634
Marusich MF, Weston JA (1992) Identification of early neurogenic cells in the neural crest lineage. Dev Biol 149:295–306
Mothe AJ, Tator CH (2005) Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience 131:177–187
Myckatyn TM, Mackinnon SE, McDonald JW (2004) Stem cell transplantation and other novel techniques for promoting recovery from spinal cord injury. Transpl Immunol 12:343–358
Namihira M, Nakashima K, Taga T (2004) Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett 572:184–188
Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S (2004) Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15:415–436
Rakic P (2007) The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res Rev 55:204–219
Schwab ME (2002) Repairing the injured spinal cord. Science 295:1029–1031
Selinfreund RH, Barger SW, Pledger WJ, Van Eldik LJ (1991) Neurotrophic protein S100 beta stimulates glial cell proliferation. Proc Natl Acad Sci USA 88:3554–3558
Siegenthaler MM, Tu MK, Keirstead HS (2007) The extent of myelin pathology differs following contusion and transection spinal cord injury. J Neurotrauma 24:1631–1646
Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A (2005) Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci 25:10–18
Stocum DL (2006) Regenerative biology and medicine. Academic Press, San Diego
Stocum DL, Zupanc GKH (2008) Stretching the limits: stem cells in regeneration science. Dev Dyn 237:3648–3671
Stuermer CAO, Bastmeyer M, Bähr M, Strobel G, Paschke K (1992) Trying to understand axonal regeneration in the CNS of fish. J Neurobiol 23:537–550
Takahashi T, Nowakowski RS, Caviness VS Jr (1993) Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci 13:820–833
Takeda A, Nakano M, Goris RC, Funakoshi K (2008) Adult neurogenesis with 5-HT expression in lesioned goldfish spinal cord. Neuroscience 151:1132–1141
Vessal M, Aycock A, Garton MT, Ciferri M, Darian-Smith C (2007) Adult neurogenesis in primate and rodent spinal cord: comparing a cervical dorsal rhizotomy with a dorsal column transection. Eur J Neurosci 26:2777–2794
Waxman SG, Anderson MJ (1986) Regeneration of central nervous system structures: Apteronotus spinal cord as a model system. In: Bullock TH, Heiligenberg W (eds) Electroreception. Wiley, New York, pp 183–208
Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M (2001) Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol 172:115–127
Yang H, Lu P, McKay HM, Bernot T, Keirstead H, Steward O, Gage FH, Edgerton VR, Tuszynski MH (2006) Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci 26:2157–2166
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
Zupanc GKH (2001) A comparative approach towards the understanding of adult neurogenesis. Brain Behav Evol 58:246–249
Zupanc GKH (2006a) Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A 192:649–670
Zupanc GKH (2006b) Adult neurogenesis and neuronal regeneration in the teleost fish brain: implication for the evolution of a primitive vertebrate trait. In: Bullock TH, Rubenstein LR (eds) The evolution of nervous systems in non-mammalian vertebrates. Academic Press, Oxford, pp 485–520
Zupanc GKH (2007) Proteomics of traumatic brain injury and regeneration. Proteomics Clin Appl 1:1362–1372
Zupanc GKH (2008) Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol (Paris) 102:357–373
Zupanc GKH (2009) Towards brain repair: insights from teleost fish. Semin Cell Dev Biol. doi:10.1016/j.semcdb.2008.12.001
Zupanc GKH, Bullock TH (2005) From electrogenesis to electroreception: an overview. In: Bullock TH, Hopkins CD, Popper AN, Fay RR (eds) Electroreception. Springer, New York, pp 5–46
Zupanc GKH, Clint SC (2003) Potential role of radial glia in adult neurogenesis of teleost fish. Glia 43:77–86
Zupanc GKH, Zupanc MM (2006) New neurons for the injured brain: mechanisms of neuronal regeneration in adult teleost fish. Regen Med 1:207–216
Zupanc GKH, Kompass KS, Horschke I, Ott R, Schwarz H (1998) Apoptosis after injuries in the cerebellum of adult teleost fish. Exp Neurol 152:221–230
Zupanc GKH, Banks JR, Engler G, Beason RC (2003) Temperature dependence of the electric organ discharge in weakly electric fish. In: Ploger BJ, Yasukawa K (eds) Exploring animal behavior in laboratory and field. Academic Press, Amsterdam, pp 85–94
Zupanc GKH, Hinsch K, Gage FH (2005) Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol 488:290–319
Zupanc GKH, Sîrbulescu RF, Nichols A, Ilies I (2006a) Electric interactions through chirping behavior in the weakly electric fish, Apteronotus leptorhynchus. J Comp Physiol A 192:159–173
Zupanc MM, Wellbrock UM, Zupanc GKH (2006b) Proteome analysis identifies novel protein candidates involved in regeneration of the cerebellum of teleost fish. Proteomics 6:677–696
Acknowledgments
This study received financial support from Wilhelm Herbst Stiftung zur Förderung von Kunst und Wissenschaft, Ernst A.-C. Lange-Stiftung, Conrad Naber Stiftung, Tönjes Vagt Stiftung, and Jacobs University Bremen. We thank U. M. Wellbrock for technical assistance and C. Ubilla and M. M. Zupanc for comments on the manuscript. All experiments were performed in accordance with the relevant German law, the Deutsches Tierschutzgesetz, of 1998. All efforts were made to reduce the number of animals used and to minimize animal suffering.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (WMV 5748 kb)
Rights and permissions
About this article
Cite this article
Sîrbulescu, R.F., Ilieş, I. & Zupanc, G.K.H. Structural and functional regeneration after spinal cord injury in the weakly electric teleost fish, Apteronotus leptorhynchus . J Comp Physiol A 195, 699–714 (2009). https://doi.org/10.1007/s00359-009-0445-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-009-0445-4