Abstract
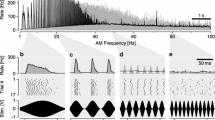
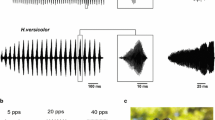
The clawed frog Xenopus laevis produces vocalizations consisting of distinct patterns of clicks. This study provides the first description of spontaneous, pure-tone and communication-signal evoked discharge properties of auditory nerve (n.VIII) fibers and dorsal medullary nucleus (DMN) cells in an obligatorily aquatic anuran. Responses of 297 n.VIII and 253 DMN units are analyzed for spontaneous rates (SR), frequency tuning, rate-intensity functions, and firing rate adaptation, with a view to how these basic characteristics shape responses to recorded call stimuli. Response properties generally resemble those in partially terrestrial anurans. Broad tuning exists across characteristic frequencies (CFs). Threshold minima are −101 dB re 1 mm/s at 675 Hz; −87 dB at 1,600 Hz; and −61 dB at 3,000 Hz (−90, −77, and −44 dB re 1 Pa, respectively), paralleling the peak frequency of vocalizations at 1.2–1.6 kHz with ∼500 Hz in 3 dB bandwidth. SRs range from 0 to 80 (n.VIII) and 0 to 73 spikes/s (DMN). Nerve and DMN units of all CFs follow click rates in natural calls, ≤67 clicks/s and faster. Units encode clicks with a single spike, double spikes, or bursts. Spike times correlate closely with click envelopes. No temporal filtering for communicative click rates occurs in either n.VIII or the DMN.










Similar content being viewed by others
Abbreviations
- ACF:
-
Autocorrelation function
- AM:
-
Amplitude modulation
- AP:
-
Amphibian papilla
- BP:
-
Basilar papilla
- CC:
-
Cross-correlation maximum
- CCF:
-
Cross-correlation function
- CF:
-
Characteristic frequency
- dB:
-
Decibels
- DMN:
-
Dorsal medullary nucleus
- DPOAE:
-
Distortion product otoacoustic emissions
- FTC:
-
Frequency–threshold curve
- n.VIII:
-
Auditory nerve
- PSTH:
-
Poststimulus time histogram
- Q10 :
-
Tuning quality factor
- SR:
-
Spontaneous rate
- TS:
-
Torus semicircularis
References
Bentley PJ, Shield JW (1973) Respiration of some urodele and anuran amphibia part 2: in air role of the skin and lungs. Comp Biochem Physiol A 46:29–38
Bibikov NG, Elepfandt A (2005) Auditory evoked potentials from medulla and midbrain in the clawed frog, Xenopus laevis laevis. Hear Res 204:29–36
Bodnar DA, Capranica RR (1994) Encoding of phase spectra by the peripheral auditory system of the bullfrog. J Comp Physiol A 174:157–171
Capranica RR (1976) Morphology and physiology of the auditory system. In: Llinás R, Precht W (eds) Frog neurobiology: a handbook. Springer, New York, pp 551–575
Capranica RR, Moffat AJ (1977) Place mechanism underlying frequency analysis in the toad’s inner ear. J Acoust Soc Am 62:S36
Christensen-Dalsgaard J, Narins PM (1993) Sound and vibration sensitivity of VIIIth nerve fibers in the frogs Leptodactylus albilabris and Rana pipiens pipiens. J Comp Physiol A 172:653–662
Christensen-Dalsgaard J, Elepfandt A (1995) Biophysics of underwater hearing in the clawed frog, Xenopus laevis. J Comp Physiol A 176:317–324
Christensen-Dalsgaard J, Jørgensen MB, Kanneworff M (1998) Basic response characteristics of auditory nerve fibers in the grassfrog (Rana temporaria). Hear Res 119:155–163
Christensen-Dalsgaard J, Kanneworff M (2005) Binaural interaction in the frog dorsal medullary nucleus. Brain Res Bull 66:522–525
Edwards CJ, Kelley DB (2001) Auditory and lateral line inputs to the midbrain of an aquatic anuran: neuroanatomic studies in Xenopus laevis. J Comp Neurol 438:148–162
Ehret G, Moffat AJ, Capranica RR (1983) Two-tone suppression in auditory nerve fibers of the green treefrog (Hyla cinerea). J Acoust Soc Am 73:2093–2095
Elepfandt A (1996) Underwater acoustics and hearing in the clawed frog, Xenopus. In: Kobel HR, Tinsley R (eds) The biology of Xenopus, Symp. Zool. Soc. London, London, vol 68, pp 177–193
Elepfandt A, Eistetter I, Fleig A, Gunther E, Hainich M, Hepperle S, Traub B (2000) Hearing threshold and frequency discrimination in the purely aquatic frog Xenopus laevis (Pipidae): measurement by means of conditioning. J Exp Biol 203(Pt 23):3621–3629
Elliott TM (2007) The neural basis of click rate coding in the auditory system. Doctor of Philosophy, Graduate School of Arts and Sciences, Columbia University, New York, p. 145
Elliott TM, Kelley DB (2007) Male discrimination of receptive and unreceptive female calls by temporal features. J Exp Biol 210:2836–2842
Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC (2004) A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol 33:197–213
Fay RR (1988) Hearing in vertebrates: a psychophysics databook. Hill-Fay, Winnetka
Fay RR, Simmons AM (1999) The sense of hearing in fishes and amphibians. In: Fay RR, Popper AN (eds) Comparative hearing: fish and amphibians, vol 11. Springer, New York, pp 269–318
Feng AS, Narins PM, Capranica RR (1975) Three populations of primary auditory fibers in the bullfrog (Rana catesbeiana): their peripheral origins and frequency sensitivities. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 100:221–229
Feng AS, Capranica RR (1976) Sound localization in anurans. I. Evidence of binaural interaction in dorsal medullary nucleus of bullfrogs (Rana catesbeiana). J Neurophysiol 39:871–881
Feng AS (1982) Quantitative analysis of intensity-rate and intensity-latency functions in peripheral auditory nerve fibers of northern leopard frogs (Rana p. pipiens). Hear Res 6:241–246
Feng AS, Hall JC, Siddique S (1991) Coding of temporal parameters of complex sounds by frog auditory nerve fibers. J Neurophysiol 65:424–445
Feng AS, Lin WY (1994) Phase-locked response characteristics of single neurons in the frog “cochlear nucleus” to steady-state and sinusoidal-amplitude-modulated tones. J Neurophysiol 72:2209–2221
Feng AS, Schellart NAM (1999) Central auditory processing in fish and amphibians. In: Fay RR, Popper AN (eds) Comparative hearing: fish and amphibians, vol 11. Springer, New York, pp 218–268
Frishkopf LS, Geisler CD (1966) Peripheral origin of auditory responses recorded from the eighth nerve of the bullfrog. J Acoust Soc Am 40:469–472
Fuzessery ZM, Feng AS (1981) Frequency representation in the dorsal medullary nucleus of the leopard frog, Rana p. pipiens. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 143:339–347
Gerhardt HC, Schwartz JJ (2001) Auditory tuning and frequency preferences in anurans. In: Ryan MJ (ed) Anuran communication, Smithsonian Institution Press, Washington, pp 73–85
Gerhardt HC, Huber F (2002) Acoustic communication in insects and anurans. University of Chicago Press, Chicago
Hall JC, Feng AS (1988) Influence of envelope rise time on neural responses in the auditory system of anurans. Hear Res 36:261–276
Hall JC, Feng AS (1990) Classification of the temporal discharge patterns of single auditory neurons in the dorsal medullary nucleus of the northern leopard frog. J Neurophysiol 64:1460–1473
Hall JC, Feng AS (1991) Temporal processing in the dorsal medullary nucleus of the Northern leopard frog (Rana pipiens pipiens). J Neurophysiol 66:955–973
Hetherington TE, Lombard RE (1982) Biophysics of underwater hearing in anuran amphibians. J Exp Biol 98:49–66
Imaizumi K, Pollack GS (2001) Neural representation of sound amplitude by functionally different auditory receptors in crickets. J Acoust Soc Am 109:1247–1260
Jørgensen MB, Christensen-Dalsgaard J (1997) Directionality of auditory nerve fiber responses to pure tone stimuli in the grassfrog, Rana temporaria. I. Spike rate responses. J Comp Physiol A 180:493–502
Kanneworff M (2004) Neural processing of directional cues in the frog dorsal medullary nucleus. PhD, Center for Sound Communication, Institute of Biology, Faculty of Science and Engineering, University of Southern Denmark, Odense, Denmark, p 100
Katbamna B, Brown JA, Collard M, Ide CF (2006) Auditory brainstem responses to airborne sounds in the aquatic frog Xenopus laevis: correlation with middle ear characteristics. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192:381–387
Kelley DB, Tobias ML (1999) Vocal communication in Xenopus laevis. In: Hauser MD, Konishi M (eds) The design of animal communication. MIT, Cambridge, pp 9–35
King BM, Minium EW (2003) Statistical reasoning in psychology and education. Wiley, Hoboken
Klump GM, Benedix JH Jr, Gerhardt HC, Narins PM (2004) AM representation in green treefrog auditory nerve fibers: neuroethological implications for pattern recognition and sound localization. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190:1011–1021
Levitt H (1971) Transformed up–down methods in psychoacoustics. J Acoust Soc Am 49(Suppl 2):467
Lombard RE, Fay RR, Werner YL (1981) Underwater hearing in the frog, Rana catesbeiana. J Exp Biol 91:57–71
Louage DH, van der Heijden M, Joris PX (2004) Temporal properties of responses to broadband noise in the auditory nerve. J Neurophysiol 91:2051–2065
Lowe DA (1986) Organisation of lateral line and auditory areas in the midbrain of Xenopus laevis. J Comp Neurol 245:498–513
Megela AL, Capranica RR (1981) Response patterns to tone bursts in peripheral auditory system of anurans. J Neurophysiol 46:465–478
Megela AL, Capranica RR (1982) Differential patterns of physiological masking in the anuran auditory nerve. J Acoust Soc Am 71:641–645
Moffat AJ, Capranica RR (1974) Sensory processing in the peripheral auditory system of treefrogs (Hyla). J Acoust Soc Am 55:480
Møhl B (1968) Hearing in seals. In: Harrison R, Hubbard R, Rice C, Schusterman RJ (eds) The behavior and physiology of pinnipeds. Appleton-Century, New York, pp 172–195
Narins PM, Capranica RR (1976) Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science 192:378–380
Nizami L (2002) Estimating auditory neuronal dynamic range using a fitted function. Hear Res 167:13–27
Paton JA, Kelley DB, Sejnowski TJ, Yodlowski ML (1982) Mapping the auditory central nervous system of Xenopus laevis with 2-deoxyglucose autoradiography. Brain Res 249:15–22
Picker M (1983) Hormonal induction of the aquatic phonotactic response of Xenopus. Behaviour 84:74–90
Popper AN (1971) The effects of size on auditory capacities of the goldfish. J Audit Res 11:239–247
Rogers PH, Cox M (1988) Underwater sound as a biological stimulus. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 131–149
Rose GJ, Capranica RR (1985) Sensitivity to amplitude modulated sounds in the anuran auditory nervous system. J Neurophysiol 53:446–465
Schwartz JJ, Simmons AM (1990) Encoding of a spectrally-complex communication sound in the bullfrog’s auditory nerve. J Comp Physiol A 166:489–499
Stevens CW, Klopp AJ, Facello JA (1994) Analgesic potency of mu and kappa opioids after systemic administration in amphibians. J Pharmacol Exp Ther 269:1086–1093
Taberner AM, Liberman MC (2005) Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93:557–569
Tobias ML, Viswanathan SS, Kelley DB (1998) Rapping, a female receptive call, initiates male–female duets in the South African clawed frog. Proc Natl Acad Sci U S A 95:1870–1875
Tobias ML, Barnard C, O’Hagan R, Horng SH, Rand M, Kelley DB (2004) Vocal communication between male Xenopus laevis. Anim Behav 67:353–365
Tougaard J (1996) Energy detection and temporal integration in the noctuid A1 auditory receptor. J Comp Physiol A 178:669–677
Trueb L (1996) Historical constraints and morphological novelties in the evolution of the skeletal system of pipid frogs (Anura: Pipidae). In: Tinsley R, Kobel R (eds) The biology of Xenopus, Clarendon, Oxford, pp 349–377
van Dijk P, Lewis ER, Wit HP (1990) Temperature effects on auditory nerve fiber response in the American bullfrog. Hear Res 44:231–240
van Dijk P, Mason M, Narins P (2002) Distortion product otoacoustic emissions in frogs: correlation with middle and inner ear properties. Hear Res 173:100
van Stokkum IH (1987) Sensitivity of neurons in the dorsal medullary nucleus of the grassfrog to spectral and temporal characteristics of sound. Hear Res 29:223–235
Vignal C, Kelley DB (2007) Significance of temporal and spectral acoustic cues for sexual recognition in Xenopus laevis. Proc R Soc Lond B Biol Sci 274:479–488
Wever EG (1985) The amphibian ear. Princeton University Press, Princeton
Will U, Fritzsch B (1988) The eighth nerve of amphibians. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 159–183
Yager DD (1992) Underwater acoustic communication in the African pipid frog Xenopus borealis. Bioacoustics 4:1–24
Yates GK, Winter IM, Robertson D (1990) Basilar membrane nonlinearity determines auditory nerve rate-intensity functions and cochlear dynamic range. Hear Res 45:203–219
Zakon HH, Wilczynski W (1988) The physiology of the anuran eighth nerve. In: Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 125–155
Acknowledgments
We gratefully acknowledge the help of Morten Kanneworff, Elizabeth Olson, Daniel Salzman, David Vicario, and Sarah Woolley. Martha Tobias made the underwater call recordings used as stimuli. We also thank two anonymous referees for comments, which strengthened the paper. Research and international travel was supported in part by a National Science Foundation graduate fellowship, by a National Institute of Deafness and Communication Disorders NRSA graduate fellowship, by NIH grant NS23684 and by the Danish National Science Foundation. Animal care and use for this study was approved both by the Institutional Animal Care and Use Committee at Columbia University (AC-AAAA1401) and the Danish Animal Experimentation Board (Dyreforsøgstilsynet), grant 1999/561–169. The experiments are legal in both the United States and Denmark, and comply with the “Principles of animal care”, publication No. 86–23, of the National Institute of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, T.M., Christensen-Dalsgaard, J. & Kelley, D.B. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis . J Comp Physiol A 193, 1243–1257 (2007). https://doi.org/10.1007/s00359-007-0285-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-007-0285-z