Abstract
Anesthetized clawed frogs (Xenopus laevis) were stimulated with underwater sound and the tympanic disk vibrations were studied using laser vibrometry. The tympanic disk velocities ranged from 0.01 to 0.5 mm/s (at a sound pressure of 2 Pa) in the frequency range of 0.4–4 kHz and were 20–40 dB higher than those of the surrounding tissue. The frequency response of the disk had two peaks, in the range of 0.6–1.1 kHz and 1.6–2.2 kHz, respectively. The first peak corresponded to the peak vibrations of the body wall overlying the lung. The second peak matched model predictions of the pulsations of the air bubble in the middle ear cavity. Filling the middle ear cavity with water lowered the disk vibrations by 10–30 dB in the frequency range of 0.5–3 kHz.
Inflating the lungs shifted the low-frequency peak downwards, but did not change the high-frequency peak. Thus, the disk vibrations in the frequency range of the mating call (main energy at 1.7–1.9 kHz) were mainly caused by pulsations of the air in the middle ear cavity; sound transmission via the lungs was more important at low frequencies (below 1 kHz). Furthermore, the low-frequency peak could be reversibly reduced in amplitude by loading the larynx with metal or tissue glue. This shows that the sound-induced vibrations of the lungs are probably coupled to the middle ear cavities via the larynx. Also, anatomical observations show that the two middle ear cavities and the larynx are connected in an air-filled recess in submerged animals.
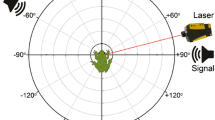
This arrangement is unique to pipid frogs and may be a structural adaptation to connect all the air spaces of the frog and improve low-frequency underwater hearing. Another function of the recess may be to allow cross-talk between the two middle ear cavities. Thus, the ear might be directional. Our pilot experiments show up to 10 dB difference between ipsi- and contralateral stimulus directions in a narrow frequency range around 2 kHz.
Similar content being viewed by others
References
Alexander R McN (1966) Physical aspects of swimbladder function. Biol Rev 41: 141–176
Christensen-Dalsgaard J, Breithaupt T, Elepfandt A (1990) Underwater hearing in the clawed frog, Xenopus laevis. Naturwissenschaften 77: 135–37
Christensen-Dalsgaard J, Elepfandt A (1992) Biophysics of under-water hearing in the clawed frog, Xenopus laevis. In: Webster DB et al. (eds) Evolutionary biology of hearing. Springer, Berlin Heidelberg New York, p 459
Cox PH, Rogers M (1988) Underwater sound as a biological stimulus. In: Atema J et al. (eds) Sensory biology of aquatic animals. Springer, Berlin Heidelberg New York, pp 131–149
Eggermont JJ (1988) Mechanisms of sound localization in anurans. In: Fritzsch B et al. (eds) The evolution of the amphibian auditory system. Wiley, New York, pp 307–336
Ehret G, Tautz J, Schmitz B, Narins PM (1990) Hearing through the lungs: transmission of sound in the frog Eleutherodactylus coqui. Naturwissenschaften 77: 192–194
Elepfandt A, Wiedemer (1987) Lateral line responses to water surface waves in the clawed frog, Xenopus laevis. J Comp Physiol A 160: 667–682
Fritzsch B (1992) The water-to-land transition: Evolution of the tetrapod basilar papilla, middle ear, and auditory nuclei. In: Webster DB et al. (eds) The evolutionary biology of hearing. Springer, Berlin Heidelberg New York, pp 351–375
Hetherington TE, Lombard RE (1982) Biophysics of underwater hearing in anuran amphibians. J Exp Biol 98: 49–66
Jørgensen MB, Schmitz B, Christensen-Dalsgaard J (1991) Biophysics of directional hearing in the frog Eleutherodactylus coqui. J Comp Physiol A 168: 223–232
Licht LE (1969) Comparative breeding behaviour of the red-legged frog (Rana aurora aurora) and the western spotted frog (Rana pretiosa pretiosa) in southwestern British Columbia. Can J Zool 47:1287–1299
Lombard RE, Fay RR, Werner YL (1981) Underwater hearing in the frog, Rana catesbeiana. J Exp Biol 91: 57–71
Michelsen A, Jørgensen MB, Christensen-Dalsgaard J, Capranica RR (1986) Directional hearing of awake, unrestrained treefrogs. Naturwissenschaften 73: 682
Møhl B (1968) Auditory sensitivity of the common seal in air and water. J Aud Res 8: 27–38
Narins PM, Hillery CM (1983) Frequency coding in the inner ear of anuran amphibians. In: Klinke R, Hartmann R (eds) Hearing — Physiological basis and psychophysics. Springer, Berlin Heidelberg New York, pp 70–76
Narins PM, Ehret G, Tautz J (1988) Acessory pathway for sound transfer in a neotropical frog. Proc Natl Acad Sci USA. 85: 1508–1512
Picker MD (1983) Hormonal induction of the aquatic phonotactic response of Xenopus. Behaviour 84: 74–90
Platt C, Popper AN (1981) Fine structure and function of the ear. In: Tavolga WN et al. (eds) Hearing and sound communication in fishes. Springer Berlin Heidelberg New York, pp 3–38
Schanz B, Elepfandt A (1988) Untersuchungen zur Ortung von Unterwasserschall durch den Krallenfrosch. Verh Dtsch Zool Ges 81: 211–212
Schuijf A (1981) Models of acoustic localization. In: Tavolga WN et al. (eds), op cit Hearing and sound communication in fishes. Springer Berlin Heidelberg New York, pp 267–310
Urick RJ (1983) Principles of underwater sound, 3rd ed. McGraw-Hill Book Comp, New York Hamburg London, p 251
Van Bergeijk WA (1966) Evolution of the sense of hearing in vertebrates. Am Zool 6: 371–377
Vigny C (1979) The mating call of 12 species and subspecies of the genus Xenopus. J Zool 1: 103–122
Villiers CGS de (1932) Über das Gehörskelett der aglossen Anuren. Anat Anz 74: 33–55
Vlaming MSMG, Aertsen AMHJ, Epping WJM (1984) Directional hearing in the grassfrog (Rana temporaria L.): I. Mechanical vibrations of tympanic membrane. Hearing Res 14: 191–201
Wever EG (1984) The amphibian ear. Princeton Univ Press, Princeton, pp 129–142
Yager DD (1992a) A unique sound production mechanism in the pipid anuran Xenopus borealis. Zool J Linn Soc 104: 351–375
Yager DD (1992b) Underwater acoustic communication in the african pipid frog Xenopus borealis. Bioacoustics 4: 1–24
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christensen-Dalsgaard, J., Elepfandt, A. Biophysics of underwater hearing in the clawed frog, Xenopus laevis . J Comp Physiol A 176, 317–324 (1995). https://doi.org/10.1007/BF00219057
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219057