Abstract
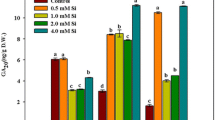
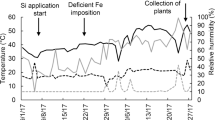
Silicon is considered an important element in several crops because it improves growth and mitigates biotic and abiotic stress. In this work, the role of silicon in limiting Fe deficiency responses by improving photosynthesis and composition of thylakoid multiprotein complexes (MPCs) has been studied. Two-week-old soybean (Glycine max L.) plants grown hydroponically with a Fe-deficient solution without FeIII-EDTA were fed with 1.0 mM silicon in the form of sodium silicate [Na2SiO3]. Responses to four Si and Fe combined treatments were analyzed after 3 and 5 days. Leaf chlorosis was generated under Fe deficiency, with decreased chlorophyll and carotenoid contents. The negative effects observed were more severe under a prolonged period of Fe deficiency. Biomass and Fe concentration in roots and shoots were largely improved by Si supply under Fe deficiency. The rates of net photosynthesis, transpiration and stomatal conductance were highly improved by the supply of Si under Fe deficiency. MPCs proteins (thylakoid complex proteins) evaluated by the first dimension followed by second-dimensional 2D-BN-SDS-PAGE and MALDI–TOF/TOF–MS observation showed that the Fe deficiency greatly decreased thylakoid MPCs and also resulted in a closure of stomata. However, these impacts of Fe deficiency were largely restored in the presence of silicon. The observed responses to silicon supply in Fe-deficient plants indicate that silicon has a significant role in limiting Fe deficiency responses by improving photosynthesis and composition of thylakoid protein complexes.









Similar content being viewed by others
References
Ali M, Farooq MA, Yasmeen T, Hussain S, Arif MS, Abbas F, Bharwana SA, Zhang G (2013) The influence of silicon on barley growth, photosynthesis and ultrastructure under chromium stress. Ecotoxicol Environ Saf 89:66–72
Amann K, Lezhneva L, Wanner G, Herrmann RG, Meurer J (2004) Accumulation of photosystem I, a member of a novel gene family is required for accumulation of [4Fe-4S] cluster-containing chloroplast complexes and antenna proteins. Plant Cell 16:3084–3097
Andaluz S, López-Milán AF, de las Rivas J, Aro EM, Abadía J, Abadía A (2006) Proteomic profiles of thylakoid membranes and changes in response to iron deficiency. Photosynth Res 89:141–155
Armstrong GA, Hearst JE (1996) Carotenoids 2: genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J 10:228–237
Arnon DI (1949) Copper enzymes in isolated chloroplast. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Astolfi S, Zuchi S, Neumann G, Cesco S, Sanita di Toppi L, Pinton R (2012) Response of barley to Fe deficiency and Cd contamination as affected by S starvation. J Exp Bot 63:1241–1250
Basa B, Lattanzio G, Solti A, Tóth B, Abadía J, Fodor F, Sárvári É (2014) Changes induced by cadmium stress and iron deficiency in the composition and organization of thylakoid complexes in sugar beet (Beta vulgaris L.). Environ Exp Bot 101:1–11
Bienfait HF, Vandenbriel W, Meslandmul NT (1985) Free space iron pools in roots generation and mobilization. Plant Physiol 78:596–600
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254
Briat JF, Celine D, Karl R, Frederic G (2010) Ferritin and iron storage in plants. Biochem Biophys Acta 1800:806–814
Cunha KPV, do Nascimento CWA, da Silva AJ (2008) Silicon alleviates the toxicity of cadmium and zinc for maize (Zea mays L.) grown on contaminated soil. J Plant Nutr Soil Sci 171:849–853
de Melo S, Monteiro F, De Bona F (2010) Silicon distribution and accumulation in shoot tissue of the tropical forage grass Brachiaria brizantha. Plant Soil 336(1):241–249
Dekker JP, Boekema EJ (2005) Supramolecular organization of thylakoid membrane proteins in green plants. Biochem Biophys Acta 1706:12–39
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci Food Agric 90:925–937
Gonzalo MJ, Lucena JJ, Hernández-Apaolaza L (2013) Effect of silicon addition on soybean (Glycine max) and cucumber (Cucumis sativus) plants grown under iron deficiency. Plant Physiol Biochem 70:455–461
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Kügler M, Jansch L, Kruft V, Schmitz UK, Braun HP (1997) Analysis of the chloroplast protein complexes by blue-native polyacrylamide gel electrophoresis (BN-PAGE). Photosynth Res 53:35–44
Liang YC, Yang CG, Shi HH (2001) Effects of silicon on growth and mineral composition of barley growth under toxic levels of aluminum. J Plant Nutr 24:229–243
Liang YC, Wong JWC, Wei L (2005) Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere 58:475–483
Liang YC, Sun WC, Zhu YG, Christie P (2007) Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut 147:422–428
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Ma JF, Tamai N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440:688–691
Marschner H (1986) Mineral nutrition of higher plants. Academic Press, London
Marschner H, Römheld V (1994) Strategies of plants for acquisition of iron. Plant Soil 165:261–274
Meda AR, Scheuermann EB, Prechsl UE, Erenoglu B, Schaaf G, Hayen H, Weber G, Wiren NV (2007) Iron acquisition by phytosiderophores contributes to cadmium tolerance. Plant Physiol 143:1761–1773
Mori S, Nishizawa N (1987) Methionine as a dominant precursor of phytosiderophores in Graminea plants. Plant Cell Physiol 28:1081–1092
Mortz E, Krogh TN, Vorum H, Görg A (2001) Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics 1:1359–1363
Muneer S, Kim TH, Qureshi MI (2012) Fe modulates Cd-induced oxidative stress and the expression of stress response proteins in the nodules of Vigna radiata. Plant Growth Regul 68:421–433
Muneer S, Ahmad J, Qureshi MI (2013a) Involvement of Fe nutrition in modulating oxidative stress and the expression of stress response proteins in leaves of Vigna radiata L. Aust J Crop Sci 7(9):1333–1342
Muneer S, Lee BR, Bae DW, Kim TH (2013b) Changes in expression of proteins involved in alleviation of Fe-deficiency by sulfur nutrition in Brassica napus L. Acta Physiol Plant 35:3037–3045
Muneer S, Lee BR, Kim KY, Park SH, Zhang Q, Kim TH (2014a) Involvement of sulphur nutrition in modulating iron deficiency responses in photosynthetic organelles of oilseed rape (Brassica napus L.). Photosynth Res 119:319–329
Muneer S, Park YG, Manivannan A, Soundararajan P, Jeong BR (2014b) Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int J Mol Sci 15(12):21803–21824
Pestana M, De Varennes A, AbadÍa J, Faria EA (2005) Differential tolerance to iron deficiency of citrus root-stocks grown in nutrient solution. Sci Hortic 104:25–36
Qureshi MI, Amici GMD, Fagioni M, Rinalducci S, Zolla L (2010) Iron stabilizes thylakoid protein-pigment complexes in Indian mustard during Cd-phytoremediation as revealed by BN-SDS-PAGE and ESI-MS/MS. J Plant Physiol 167:761–770
Rizwan M, Meunier JD, Miche H, Keller C (2012) Effect of silicon on reducing cadmium toxicity in durum wheat (Triticum turgidum L. cv. Claudio W.) grown in a soil with aged contamination. J Hazard Mater 209–210:326–334
Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins from silver stained polyacrylamide gels. Anal Chem 68:850–858
Song AL, Li ZJ, Zhang J, Xue GF, Fan FL, Liang YC (2009) Silicon-enhanced resistance to cadmium toxicity in Brassica chinensis L. is attributed to Si-suppressed cadmium uptake and transport and Si-enhanced antioxidant defense capacity. J Hazard Mater 172:74–83
Szacilowski K, Chmura A, Stasicka Z (2005) Interplay between iron complexes, nitric oxide and sulfur ligands: structure, (photo) reactivity and biological importance. Coord Chem Rev 249:2408–2436
Timperio AM, D’ Amici GM, Barta C, Loreto F, Zolla L (2007) Proteomics, pigment composition, and organization of thylakoid membranes in iron deficient spinach leaves. J Exp Bot 58:3695–3710
Vaculìk M, Lux A, Luxová M, Tanimoto E, Lichtscheidl I (2009) Silicon mitigates cadmium inhibitory effects in young maize plants. Environ Exp Bot 67:52–58
Vert G, Natasha G, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from soil and for plant growth. Plant Cell 14:1223–1233
Wang L, Wang W, Chen Q, Cao W, Li M, Zhang F (2000) Silicon induced cadmium tolerance of rice seedlings. J Plant Nutr 23:1397–1406
Waters BM, Blevins DG, Eide DJ (2002) Characterization of FRO1, a Pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol 129:85–94
Xu Z, Zhou G (2008) Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J Exp Bot 59(12):3317–3325
Yoshihara T, Hodoshima H, Miyano Y, Shoji K, Shimada H, Goto F (2006) Cadmium inducible Fe deficiency responses observed from macro and molecular views in tobacco plants. Plant Cell Rep 25:365–373
You-Qiang F, Hong S, Dao-Ming W, Kun-Zheng C (2012) Silicon-mediated amelioration of Fe2+ toxicity in rice (Oryza sativa L.) roots. Pedosphere 22:795–802
Zhang YP, Wang ZM, Wu YC, Zhang X (2006) Stomatal characteristics of different green organs in wheat under different irrigation regimes. Acta Agric Sinica 32:70–75
Zuchi S, Cesco S, Astolfi S (2012) High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Environ Exp Bot 77:25–32
Acknowledgments
This work was supported by Bk21 Plus program, Gyeongsang National University, Korea. The authors would like to thank Professor Jong Il Chung, Department of Agronomy, Gyeongsang National University for providing the soybean seeds. Authors also thank Central Instrumentation Facility, Gyeongsang National University, Korea for assistance in carrying out mass spectrometry.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muneer, S., Jeong, B.R. Silicon Decreases Fe Deficiency Responses by Improving Photosynthesis and Maintaining Composition of Thylakoid Multiprotein Complex Proteins in Soybean Plants (Glycine max L.). J Plant Growth Regul 34, 485–498 (2015). https://doi.org/10.1007/s00344-015-9484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9484-y