Abstract
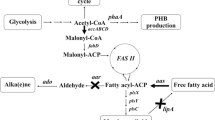
Genetic modification is useful for improving the nutritional qualities of cyanobacteria. To increase the total unsaturated fatty acid content, along with the ratio of ω-3/ω-6 fatty acids, genetic engineering can be used to modify fatty acid metabolism. Synechococcus sp. PCC7002, a fast-growing cyanobacterium, does not contain a Δ6 desaturase gene and is therefore unable to synthesize γ-linolenic acid (GLA) and stearidonic acid (SDA), which are important in human health. In this work, we constructed recombinant vectors Syd6D, Syd15D and Syd6Dd15D to express the Δ15 desaturase and Δ6 desaturase genes from Synechocystis PCC6803 in Synechococcus sp. PCC7002, with the aim of expressing polyunsaturated fatty acids. Overexpression of the Δ15 desaturase gene in Synechococcus resulted in 5.4 times greater accumulation of α-linolenic acid compared with the wild-type while Δ6 desaturase gene expression produced both GLA and SDA. Co-expression of the two genes resulted in low-level accumulation of GLA but much larger amounts of SDA, accounting for as much to 11.64% of the total fatty acid content.
Similar content being viewed by others
References
Bakowska-Barczak A M, Schieber A, Kolodziejczyk P. 2009. Characterization of Canadian black currant (Ribes nigrum L.) seed oils and residues. J. Agric. Food Chem., 57 (24): 11528–11536.
Banz W J, Davis J E, Clough R W, Cheatwood J L. 2012. Stearidonic acid: is there a role in the prevention and management of type 2 diabetes mellitus? J. Nutr., 142 (3): 635S–640S.
Becker E W. 1994. Microalgae: Biotechnology and Microbiology. Cambridge University Press, Cambridge. 293p.
Bernstein H C, Konopka A, Melnicki M R, Hill E A, Kucek L A, Zhang S Y, Shen G Z, Bryant D A, Beliaev A S. 2014. Effect of mono-and dichromatic light quality on growth rates and photosynthetic performance of Synechococcus sp. PCC 7002. Front Microbiol., 5: 488.
Bligh E G, Dyer W J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol., 37 (8): 911–917.
Boyanapalli R, Bullerjahn G S, Pohl C, Croot P L, Boyd P W, McKay R M L. 2007. Luminescent whole-cell cyanobacterial bioreporter for measuring Fe availability in diverse marine environments. Appl. Environ. Microbiol., 73 (3): 1019–1024.
Chen G, Qu S J, Wang Q, Bian F, Peng Z Y, Zhang Y, Ge H T, Yu J H, Xuan N, Bi Y P, He Q F. 2014. Transgenic expression of delta-6 and delta-15 fatty acid desaturases enhances omega-3 polyunsaturated fatty acid accumulation in Synechocystis sp. PCC6803. Biotechnol. Biofuels, 7: 32.
Davidson M H. 2013. Omega-3 fatty acids: new insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid. Curr. Opin. Lipidol., 24 (6): 467–474.
Davies F K, Work V H, Beliaev A S, Posewitz M C. 2014. Engineering limonene and bisabolene production in wild type and a glycogen-deficient mutant of Synechococcus sp. PCC 7002. Front Bioeng. Biotechnol., 2: 21.
Eckert H, La Vallee B, Schweiger B J, Kinney A J, Cahoon E B, Clemente T. 2006. Co-expression of the borage Δ 6 desaturase and the Arabidopsis Δ 15 desaturase results in high accumulation of stearidonic acid in the seeds of transgenic soybean. Planta, 224 (5): 1050–1057.
Gallardo M A, Cárcamo J G, Hiller B, Nuernberg G, Nuernberg K, Dannenberger D. 2015. Expression of lipid metabolism related genes in subcutaneous adipose tissue from Chilota lambs grazing on two different pasture types. Eur. J. Lipid Sci. Technol., 117 (1): 23–30, http://dx.doi.org/10.1002/ ejlt.201400033.
Graham I A, Larson T, Napier J A. 2007. Rational metabolic engineering of transgenic plants for biosynthesis of omega-3 polyunsaturates. Curr. Opin. Biotechnol., 18 (2): 142–147.
Harris W S. 2012. Stearidonic acid-enhanced soybean oil: a plant-based source of (n-3) fatty acids for foods. J. Nutr., 142 (3): 600S–604S.
Jacobsen J H, Frigaard N U. 2014. Engineering of photosynthetic mannitol biosynthesis from CO2 in a cyanobacterium. Metab. Eng., 21: 60–70.
Kenyon C N. 1972. Fatty acid composition of unicellular strains of blue-green algae. J. Bact eriol., 109 (2): 827–834.
Khozin-Goldberg I, Iskandarov U, Cohen Z. 2011. LC-PUFA from photosynthetic microalgae: occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol., 91 (4): 905–915.
Kim S H, Park J S, Kim S Y, Kim J B, Roh K H, Kim H U, Lee K R, Kim J B. 2014. Functional characterization of polyunsaturated fatty acid delta 6-desaturase and elongase genes from the black seabream (Acanthopagrus schlegelii). Cell Biochem. Biophys., 68 (2): 335–346.
Lazic M, Inzaugarat M E, Povero D, Zhao I C, Chen M, Nalbandian M, Miller Y I, Cherñ avsky A C, Feldstein A E, Sears D D. 2014. Reduced dietary omega-6 to omega-3 fatty acid ratio and 12/15-lipoxygenase deficiency are protective against chronic high fat diet-induced steatohepatitis. PLoS One, 9 (9): e107658.
Lee J H, O’Keefe J H, Lavie C J, Harris W S. 2009. Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat. Rev. Cardiol., 6 (12): 753–758.
Lenihan-Geels G, Bishop K S, Ferguson L R. 2013. Alternative sources of omega-3 fats: can we find a sustainable substitute for fish? Nutrients, 5 (4): 1301–1315.
Liu X, Sheng J, Curtiss R III. 2011. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA, 108(17): 6 899–6 904.
Maslova I P, Muradyan E A, Lapina S S, Klyachko-Gurvich G L, Los D A. 2004. Lipid fatty acid composition and thermophilicity of cyanobacteria. Russ. J. Plant Physiol., 51(3): 353–360.
McNeely K, Xu Y, Bennette N, Bryant D A, Dismukes G C. 2010. Redirecting reductant flux into hydrogen production via metabolic engineering of fermentative carbon metabolism in a cyanobacterium. Appl. Environ. Microbiol., 76 (15): 5032–5038.
Meesapyodsuk D, Qiu X. 2012. The front-end desaturase: structure, function, evolution and biotechnological use. Lipids, 47 (3): 227–237.
Möllers K B, Cannella D, Jørgensen H, Frigaard N U. 2014. Cyanobacterial biomass as carbohydrate and nutrient feedstock for bioethanol production by yeast fermentation. Biotechnol. Biofuels, 7: 64.
Murata N, Deshnium P, Tasaka Y. 1996. Biosynthesis of gamma-linolenic acid in the cyanobacterium Spirulina platensis, in γ-linolenic acid. In: Huang Y S, Milles D E eds. AOCS Press, Champaign. p.22–32.
Murata N, Wada H. 1995. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem. J., 308 (1): 1–8.
Nakamura Y, Kaneko T, Hirosawa M, Miyajima N, Tabata S. 1998. CyanoBase, a www database containing the complete nucleotide sequence of the genome of Synechocystis sp. strain PCC6803. Nucl. Acids Res., 26 (1): 63–67.
Reddy A S, Nuccio M L, Gross L M, Thomas T L. 1993. Isolation of a Δ 6 -desaturase gene from the cyanobacterium synechocystis sp. strain PCC 6803 by gain-of-function expression in Anabaena sp. strain PCC 7120. Plant Mol. Biol., 22 (2): 293–300.
Reddy A S, Thomas T L. 1996. Expression of a cyanobacterial Δ 6 -desaturase gene results in γ-linolenic acid production in transgenic plants. Nat. Biotechnol., 14 (5): 639–642.
Reed D W, Schafer U A, Covello P S. 2000. Characterization of the Brassica napus extraplastidial linoleate desaturase by expression in Saccharomyces cerevisiae. Plant Physiol., 122 (3): 715–720.
Sato S, Xing A Q, Ye X G, Schweiger B, Kinney A, Graef G, Clemente T. 2004. Production of γ-linolenic acid and stearidonic acid in seeds of marker-free transgenic soybean. Crop Sci., 44 (2): 646–652.
Sayanova O V, Beaudoin F, Michaelson L V, Shewry P R, Napier J A. 2003. Identification of primula fatty acid delta 6-desaturases with n-3 substrate preferences. FEBS Lett., 542 (1-3): 100–104.
Simon D, Eng P A, Borelli S, Kägi R, Zimmermann C, Zahner C, Drewe J, Hess L, Ferrari G, Lautenschlager S, Wü thrich B, Schmid-Grendelmeier P. 2014. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv. Ther., 31 (2): 180–188.
Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. 1971. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev., 35 (2): 171–205.
Stevens S E Jr, Patterson C O P, Myers J. 1973. The production of hydrogen peroxide by blue-green algae: a survey. J. Phycol., 9 (4): 427–430.
Takeyama H, Takeda D, Yazawa K, Yamada A, Matsunaga T. 1997. Expression of the eicosapentaenoic acid synthesis gene cluster from Shewanella sp. in a transgenic marine cyanobacterium, Synechococcus sp. Microbiology, 143(8): 2 725–2 731.
Tan L, Meesapyodsuk D, Qiu X. 2011. Molecular analysis of Δ6 desaturase and Δ6 elongase from Conidiobolus obscurus in the biosynthesis of eicosatetraenoic acid, a ω3 fatty acid with nutraceutical potentials. Appl. Microbiol. Biotechnol., 90 (2): 591–601.
Tasset-Cuevas I, Fernández-Bedmar Z, Lozano-Baena M D, Campos-Sánchez J, de Haro-Bailón A, Muñoz-Serrano A, Alonso-Moraga Á. 2013. Protective effect of borage seed oil and gamma linolenic acid on DNA: in vivo and in vitro studies. PLoS One, 8 (2): e56986.
Therien J B, Zadvornyy O A, Posewitz M C, Bryant D A, Peters J W. 2014. Growth of Chlamydomonas reinhardtii in acetate-free medium when co-cultured with alginateencapsulated, acetate-producing strains of Synechococcus sp. PCC 7002. Biotechnol. Biofuels, 7: 154.
Ursin V M. 2003. Modification of plant lipids for human health: development of functional land-based omega-3 fatty acids. J. Nutr., 133 (12): 4271–4274.
Wang H S, Yu C, Tang X F, Zhu Z J, Ma N N, Meng Q W. 2014. A tomato endoplasmic reticulum (ER)-type omega-3 fatty acid desaturase (LeFAD3) functions in early seedling tolerance to salinity stress. Plant Cell Rep., 33 (1): 131–142.
Yu C H, Wang H S, Yang S, Tang X F, Duan M, Meng Q W. 2009. Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. Plant Physiol. Biochem., 47 (11-12): 1 102–1 112.
Zhang M, Barg R, Yin M G, Gueta-Dahan Y, Leikin-Frenkel A, Salts Y, Shabtai S, Ben-Hayyim G. 2005. Modulated fatty acid desaturation via overexpression of two distinct ω-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J., 44 (3): 361–371.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the International S&T Cooperation Program of China (No. 2012DFA30450), the National Natural Science Foundation of China (No. 30871541), the Taishan Scholar Foundation of Shandong Province (No. tshw20091014), and the Innovation Program of the University Institutes of Jinan, Shandong Province (No. 201004044)
Rights and permissions
About this article
Cite this article
Dong, X., He, Q., Peng, Z. et al. Production of γ-linolenic acid and stearidonic acid by Synechococcus sp. PCC7002 containing cyanobacterial fatty acid desaturase genes. Chin. J. Ocean. Limnol. 34, 772–780 (2016). https://doi.org/10.1007/s00343-016-4369-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00343-016-4369-x