Abstract
Objectives
The purpose of the study was to explore the performance of a three-component diffusion model in evaluating the degree of malignancy and isocitrate dehydrogenase 1 (IDH-1) gene type of gliomas.
Methods
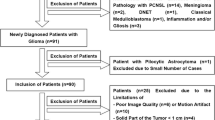
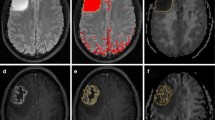
Overall, 60 patients with gliomas were enrolled. The intermediate and perfusion-related diffusion coefficients (Dint and Dp) and fractions of strictly limited, intermediate, and perfusion-related diffusion (Fvery-slow, Fint, and Fp) were obtained with a three-component diffusion model. Parameters were also obtained from a diffusion kurtosis model and mono- and biexponential models. All parameters were compared between different tumor grades and IDH-1 gene types. Diagnostic performance and logistic regression analyses were performed.
Results
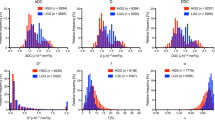
High-grade gliomas (HGGs) had significantly higher Fint, Fvery-slow, and Dp values but significantly lower Fp and Dint values than low-grade gliomas (LGGs), and Fint and Fp differed significantly among grade II, III, and IV gliomas (p < 0.05 for all). Fint achieved the highest AUC of 0.872 in differentiating between LGGs and HGGs. Logistic regression analysis revealed that in each model, Fint, diffusion coefficient (D), apparent diffusion coefficient (ADC), mean diffusivity (MD), and mean kurtosis (MK) were associated with glioma grading. After multiple regression analysis, Fint remained the only differentiator. Additionally, Fint and Fp showed significant differences between IDH-1 mutated and IDH-1 wild-type gliomas (p = 0.007 and 0.01, respectively).
Conclusions
The three-component DWI model served as a useful biomarker for detecting microstructural features in gliomas with different grades and IDH-1 mutation statuses.
Key Points
• The three-component model enables the estimation of an intermediate diffusion component.
• The three-component model performed better than the other models in glioma grading and genotyping.
• Fint was useful in evaluating the grade and genotype of gliomas.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- DWI:
-
Diffusion-weighted imaging
- EES:
-
Extravascular extracellular space
- HGG:
-
High-grade glioma
- IDH-1:
-
Isocitrate dehydrogenase 1
- IVIM:
-
Intravoxel incoherent motion
- LGG:
-
Low-grade glioma
- MRI:
-
Magnetic resonance imaging
- NNLS:
-
Non-negative least squares
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- WHO:
-
World Health Organization
References
Villa C, Miquel C, Mosses D, Bernier M, Di Stefano AL (2018) The 2016 World Health Organization classification of tumours of the central nervous system. Presse Med 47:e187–e200
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Yan H, Parsons DW, Jin G et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol 188:1622–1635
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Le Bihan D, Turner R (1992) The capillary network: a link between IVIM and classical perfusion. Magn Reson Med 27:171–178
Gao A, Zhang H, Yan X et al (2022) Whole-tumor histogram analysis of multiple diffusion metrics for glioma genotyping. Radiology 302:652–661
Koh DM, Collins DJ, Orton MR (2011) Intravoxel incoherent motion in body diffusion-weighted MRI: reality and challenges. AJR Am J Roentgenol 196:1351–1361
Bisdas S, Koh TS, Roder C et al (2013) Intravoxel incoherent motion diffusion-weighted MR imaging of gliomas: feasibility of the method and initial results. Neuroradiology 55:1189–1196
Ohno N, Miyati T, Kobayashi S, Gabata T (2016) Modified triexponential analysis of intravoxel incoherent motion for brain perfusion and diffusion. J Magn Reson Imaging 43:818–823
Ueda Y, Takahashi S, Ohno N et al (2016) Triexponential function analysis of diffusion-weighted MRI for diagnosing prostate cancer. J Magn Reson Imaging 43:138–148
Wong SM, Backes WH, Drenthen GS et al (2020) Spectral diffusion analysis of intravoxel incoherent motion MRI in cerebral small vessel disease. J Magn Reson Imaging 51:1170–1180
Cercueil JP, Petit JM, Nougaret S et al (2015) Intravoxel incoherent motion diffusion-weighted imaging in the liver: comparison of mono-, bi- and tri-exponential modelling at 3.0-T. Eur Radiol 25:1541–1550
Fujima N, Sakashita T, Homma A et al (2017) Advanced diffusion models in head and neck squamous cell carcinoma patients: goodness of fit, relationships among diffusion parameters and comparison with dynamic contrast-enhanced perfusion. Magn Reson Imaging 36:16–23
Ling C, Shi F, Zhang J, Jiang B, Dong F, Zeng Q (2019) In vivo measurement of cytoplasmic organelle water fraction using diffusion-weighted imaging: application in the malignant grading and differential diagnosis of gliomas. Medicine (Baltimore) 98:e17949
Chevallier O, Zhou N, Cercueil JP, He J, Loffroy R, Wang YXJ (2019) Comparison of tri-exponential decay versus bi-exponential decay and full fitting versus segmented fitting for modeling liver intravoxel incoherent motion diffusion MRI. NMR Biomed 32:e4155
van der Thiel MM, Freeze WM, Verheggen ICM et al (2021) Associations of increased interstitial fluid with vascular and neurodegenerative abnormalities in a memory clinic sample. Neurobiol Aging 106:257–267
Mills SJ, Soh C, Rose CJ et al (2010) Candidate biomarkers of extravascular extracellular space: a direct comparison of apparent diffusion coefficient and dynamic contrast-enhanced MR imaging--derived measurement of the volume of the extravascular extracellular space in glioblastoma multiforme. AJNR Am J Neuroradiol 31:549–553
Wang Y, Zhang F, Xiong N et al (2021) Remodelling and treatment of the blood-brain barrier in glioma. Cancer Manag Res 13:4217–4232
Grant SC, Buckley DL, Gibbs S, Webb AG, Blackband SJ (2001) MR microscopy of multicomponent diffusion in single neurons. Magn Reson Med 46:1107–1112
Xueying L, Zhongping Z, Zhoushe Z et al (2015) Investigation of apparent diffusion coefficient from ultra-high b-values in Parkinson’s disease. Eur Radiol 25:2593–2600
Togao O, Hiwatashi A, Yamashita K et al (2016) Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro Oncol 18:132–141
Bai Y, Lin Y, Tian J et al (2016) Grading of gliomas by using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging and diffusion kurtosis MR imaging. Radiology 278:496–504
Jabehdar Maralani P, Myrehaug S, Mehrabian H et al (2021) Intravoxel incoherent motion (IVIM) modeling of diffusion MRI during chemoradiation predicts therapeutic response in IDH wildtype glioblastoma. Radiother Oncol 156:258–265
Lu J, Li X, Li H (2021) Perfusion parameters derived from MRI for preoperative prediction of IDH mutation and MGMT promoter methylation status in glioblastomas. Magn Reson Imaging 83:189–195
Morozov S, Sergunova K, Petraikin A et al (2020) Diffusion processes modeling in magnetic resonance imaging. Insights Imaging 11:60
Yao X, Derugin N, Manley GT, Verkman AS (2015) Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci Lett 584:368–372
Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024
Zeng Q, Shi F, Zhang J, Ling C, Dong F, Jiang B (2018) A modified tri-exponential model for multi-b-value diffusion-weighted imaging: a method to detect the strictly diffusion-limited compartment in brain. Front Neurosci 12:102
Funding
This study was funded by the National Natural Science Foundation of China (No. 82171885, 81971583); Shanghai Science and Technology Commission Explorer Program (21TS1400700); Shanghai Natural Science Foundation (20ZR1433200); and the Medical Engineering Cross Research Foundation of Shanghai Jiao Tong University (No. YG2022QN035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Yan Zhou.
Conflict of interest
The authors of this manuscript declare relationships with the following companies: MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, M., Wang, X., Liu, F. et al. A three-component multi-b-value diffusion-weighted imaging might be a useful biomarker for detecting microstructural features in gliomas with differences in malignancy and IDH-1 mutation status. Eur Radiol 33, 2871–2880 (2023). https://doi.org/10.1007/s00330-022-09212-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09212-5