Abstract
Objectives
To assess brain damage in Parkinson’s disease (PD) based on apparent diffusion coefficient (ADC) data obtained from ultra-high b-values.
Methods
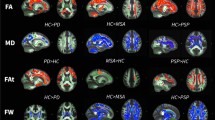
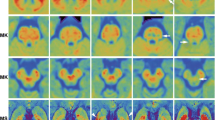
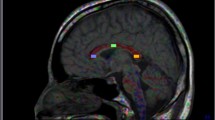
Eighteen PD patients and 18 controls received diffusion-weighted imaging (DWI) with standard b-values (0, 1,000 s/mm2) and 15 b-values (0–5,000 s/mm2). Standard ADC (ADCst) maps were calculated from standard b-values, while maps of pure diffusion coefficients (D), pseudo-diffusion coefficients (D*), and ultra-high ADCs (ADCuh) were calculated from the 15 b-values using a tri-component model. In this model, D and D* values were quantified with a bi-exponential equation using b-values less than 2,000 s/mm2, while ADCuh was quantified by fitting the signals at ultra-high b-values (2,000–5,000 s/mm2) to the mono-exponential equation. ADCst, ADCuh, D, and D* of the globus pallidus (GP), putamen (P), and substantia nigra (SN) were compared between PD patients and normal control subjects.
Results
ADCuh of the GP, P, and SN was significantly lower in PD patients than those in control subjects (P < 0.001), while ADCst, D, and D* of the GP, P and SN were not different between the two groups (P > 0.05).
Conclusions
ADCuh may be a useful measurement for evaluating brain damage in PD patients.
Key Points
• DWI with ultra-high b-values may provide new insight into Parkinson’s disease pathology
• ADC calculated using ultra-high b-values is different between PD and controls
• ADC uh may be associated with water transportation by aquaporins


Similar content being viewed by others
Abbreviations
- 3D BRAVO:
-
three-dimensional brain volume imaging
- ADC:
-
apparent diffusion coefficient
- ADCst :
-
ADC calculated using standard b-values
- ADCuh :
-
ADC calculated using ultra-high b-values
- BA:
-
Bland–Altman
- D:
-
pure diffusion coefficient
- D* :
-
pseudo-diffusion coefficient
- DWI:
-
diffusion weighted imaging
- ECS:
-
extracellular compartment space
- FOV:
-
field of view
- FSE:
-
axial fast spin-echo
- HY:
-
Hoehn and Yahr staging scale
- ICC:
-
intraclass correlation
- LOA:
-
limits of agreement
- MD:
-
mean diffusivity
- MSA:
-
multiple system atrophy
- MSA-C:
-
MSA with predominant cerebellar dysfunction
- MSA-P:
-
MSA with predominant Parkinsonism
- PD:
-
Parkinson’s disease
- ROI:
-
region of interest
- TE:
-
echo time
- TR:
-
repetition time
- T2FLAIR:
-
T2 fluid attenuation inversion recovery
- T2WI:
-
T2-weighted imaging
- UPDRS:
-
Unified Parkinson’s Disease Rating Scale
- SD:
-
standard deviation
References
Chen NC, Huang CW, Lui CC et al (2013) Diffusion-weighted imaging improves prediction in cognitive outcome and clinical phases in patients with carbon monoxide intoxication. Neuroradiology 55:107–115
Kang Y, Choi SH, Kim YJ et al (2011) Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging–correlation with tumor grade. Radiology 261:882–890
Maier SE, Sun Y, Mulkern RV (2010) Diffusion imaging of brain tumors. NMR Biomed 23:849–864
Kobayashi S, Koga F, Yoshida S et al (2011) Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol 21:2178–2186
Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168:497–505
Chiaradia M, Baranes L, Van Nhieu JT et al (2014) Intravoxel incoherent motion (IVIM) MR imaging of colorectal liver metastases: are we only looking at tumor necrosis? J Magn Reson Imaging 39:317–325
Federau C, Maeder P, O'Brien K, Browaeys P, Meuli R, Hagmann P (2012) Quantitative measurement of brain perfusion with intravoxel incoherent motion MR imaging. Radiology 265:874–881
Sigmund EE, Vivier PH, Sui D et al (2012) Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 263:758–769
Catalano OA, Choy G, Zhu A, Hahn PF, Sahani DV (2010) Differentiation of malignant thrombus from bland thrombus of the portal vein in patients with hepatocellular carcinoma: application of diffusion-weighted MR imaging. Radiology 254:154–162
Lai V, Li X, Lee VH, Lam KO, Chan Q, Khong PL (2013) Intravoxel incoherent motion MR imaging: comparison of diffusion and perfusion characteristics between nasopharyngeal carcinoma and post-chemoradiation fibrosis. Eur Radiol 23:2793–2801
Le Bihan D (2013) Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 268:318–322
Kong L, Lian G, Zheng W, Liu H, Zhang H, Chen R (2013) Effect of alcohol on diffuse axonal injury in rat brainstem: diffusion tensor imaging and aquaporin-4 expression study. Biomed Res Int 2013:798261
Yao X, Derugin N, Manley GT, Verkman AS (2015) Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci Lett 584:368–372
Katahira K, Takahara T, Kwee TC et al (2011) Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: evaluation in 201 cases with histopathological correlation. Eur Radiol 21:188–196
Bano S, Waraich MM, Khan MA, Buzdar SA, Manzur S (2013) Diagnostic value of apparent diffusion coefficient for the accurate assessment and differentiation of intracranial meningiomas. Acta Radiol Short Rep 2:2047981613512484
Degirmenci B, Yaman M, Haktanir A, Albayrak R, Acar M, Caliskan G (2007) The effects of levodopa use on diffusion coefficients in various brain regions in Parkinson's disease. Neurosci Lett 416:294–298
Tsukamoto K, Matsusue E, Kanasaki Y et al (2012) Significance of apparent diffusion coefficient measurement for the differential diagnosis of multiple system atrophy, progressive supranuclear palsy, and Parkinson's disease: evaluation by 3.0-T MR imaging. Neuroradiology 54:947–955
Chan LL, Ng KM, Rumpel H, Fook-Chong S, Li HH, Tan EK (2014) Transcallosal diffusion tensor abnormalities in predominant gait disorder parkinsonism. Parkinsonism Relat Disord 20:53–59
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Massano J, Bhatia KP (2012) Clinical approach to Parkinson's disease: features, diagnosis, and principles of management. Cold Spring Harb Perspect Med 2:a008870
Fearnley JM, Lees AJ (1991) Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114(Pt 5):2283–2301
Obeso JA, Rodriguez-Oroz MC, Rodriguez M et al (2000) Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci 23:S8–S19
Aquino D, Contarino V, Albanese A et al (2014) Substantia nigra in Parkinson's disease: a multimodal MRI comparison between early and advanced stages of the disease. Neurol Sci 35:753–758
Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP (2013) Diffusion tensor imaging of nigral degeneration in Parkinson's disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. Neuroimage Clin 3:481–488
Chan LL, Rumpel H, Yap K et al (2007) Case control study of diffusion tensor imaging in Parkinson's disease. J Neurol Neurosurg Psychiatry 78:1383–1386
Chu HH, Choi SH, Ryoo I et al (2013) Differentiation of true progression from pseudoprogression in glioblastoma treated with radiation therapy and concomitant temozolomide: comparison study of standard and high-b-value diffusion-weighted imaging. Radiology 269:831–840
Badaut J, Ashwal S, Adami A et al (2011) Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J Cereb Blood Flow Metab 31:819–831
Tourdias T, Dragonu I, Fushimi Y et al (2009) Aquaporin 4 correlates with apparent diffusion coefficient and hydrocephalus severity in the rat brain: a combined MRI-histological study. Neuroimage 47:659–666
Amiry-Moghaddam M, Lindland H, Zelenin S et al (2005) Brain mitochondria contain aquaporin water channels: evidence for the expression of a short AQP9 isoform in the inner mitochondrial membrane. FASEB J 19:1459–1467
Chi Y, Fan Y, He L et al (2011) Novel role of aquaporin-4 in CD4+ CD25+ T regulatory cell development and severity of Parkinson's disease. Aging Cell 10:368–382
Umemura A, Oeda T, Hayashi R et al (2013) Diagnostic accuracy of apparent diffusion coefficient and 123I-metaiodobenzylguanidine for differentiation of multiple system atrophy and Parkinson's disease. PLoS One 8:e61066
Nicoletti G, Lodi R, Condino F et al (2006) Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson's disease and progressive supranuclear palsy. Brain 129:2679–2687
Michaeli S, Oz G, Sorce DJ et al (2007) Assessment of brain iron and neuronal integrity in patients with Parkinson's disease using novel MRI contrasts. Mov Disord 22:334–340
Vaillancourt DE, Spraker MB, Prodoehl J et al (2009) High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson’s disease. Neurology 72:1378–1384
Hyare H, Thornton J, Stevens J et al (2010) High-b-value diffusion MR imaging and basal nuclei apparent diffusion coefficient measurements in variant and sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol 31:521–526
Acknowledgments
The scientific guarantor of this publication is Huangli. Dr. Zhang Zhongping and Dr. Zhao Zhoushe declare relationships with the following companies: General Electric Healthcare China. The study has received funding by the Guangzhou Science and Technique Program of China (No. 2012j4300077). No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all patients in this study. Methodology: prospective case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xueying, L., Zhongping, Z., Zhoushe, Z. et al. Investigation of Apparent Diffusion Coefficient from Ultra-high b-Values in Parkinson’s Disease. Eur Radiol 25, 2593–2600 (2015). https://doi.org/10.1007/s00330-015-3678-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3678-3