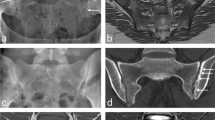
Abstract
Objectives
To evaluate the technical feasibility and applicability of quantitative MR techniques (delayed gadolinium-enhanced MRI of cartilage (dGEMRIC), T2 mapping, T2* mapping) at 7 T MRI for assessing hip cartilage.
Methods
Hips of 11 healthy volunteers were examined at 7 T MRI with an 8-channel radiofrequency transmit/receive body coil using multi-echo sequences for T2 and T2* mapping and a dual flip angle gradient-echo sequence before (T10) and after intravenous contrast agent administration (T1Gd; 0.2 mmol/kg Gd-DTPA2− followed by 0.5 h of walking and 0.5 h of rest) for dGEMRIC. Relaxation times of cartilage were measured manually in 10 regions of interest. Pearson’s correlations between R1delta = 1/T1Gd − 1/T10 and T1Gd and between T2 and T2* were calculated. Image quality and the delineation of acetabular and femoral cartilage in the relaxation time maps were evaluated using discrete rating scales.
Results
High correlations were found between R1delta and T1Gd and between T2 and T2* relaxation times (all p < 0.01). All techniques delivered diagnostic image quality, with best delineation of femoral and acetabular cartilage in the T2* maps (mean 3.2 out of a maximum of 4 points).
Conclusions
T1, T2 and T2* mapping of hip cartilage with diagnostic image quality is feasible at 7 T. To perform dGEMRIC at 7 T, pre-contrast T1 mapping can be omitted.
Key Points
• dGEMRIC of hip cartilage with diagnostic image quality is feasible at 7 T.
• To perform dGEMRIC at 7 T, pre-contrast T1 mapping can be omitted.
• T2(*) mapping of hip cartilage with diagnostic image quality is feasible at 7 T.
• T2 and T2* relaxation times of cartilage were highly correlated at 7 T.
• Best delineation of femoral and acetabular cartilage was found in T2* maps.





Similar content being viewed by others
Abbreviations
- BMI:
-
body mass index
- dGEMRIC:
-
delayed gadolinium-enhanced MRI of cartilage
- DREAM:
-
dual refocusing echo acquisition mode
- FOV:
-
field of view
- FLASH:
-
fast low angle shot
- MRI:
-
magnetic resonance imaging
- RF:
-
radiofrequency
- ROI:
-
region of interest
- SAR:
-
specific absorption rate
- SD:
-
standard deviation
- SNR:
-
signal-to-noise ratio
- T:
-
Tesla
- TI:
-
inversion time
- TE:
-
echo time
- TR:
-
repetition time
- UHF:
-
ultra-high field
References
Rogers AD, Payne JE, Yu JS (2013) Cartilage imaging: a review of current concepts and emerging technologies. Semin Roentgenol 48:148–157
Sanghvi D, Munshi M, Pardiwala D (2014) Imaging of cartilage repair procedures. Indian J Radiol Imaging 24:249–253
Mosher TJ, Walker EA, Petscavage-Thomas J, Guermazi A (2013) Osteoarthritis year 2013 in review: imaging. Osteoarthritis Cartilage 21:1425–1435
Bashir A, Gray ML, Burstein D (1996) Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med 36:665–673
Burstein D, Velyvis J, Scott KT et al (2001) Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 45:36–41
Binks DA, Hodgson RJ, Ries ME et al (2013) Quantitative parametric MRI of articular cartilage: a review of progress and open challenges. Br J Radiol 86:20120163
Kijowski R (2010) Clinical cartilage imaging of the knee and hip joints. AJR Am J Roentgenol 195:618–628
Krug R, Stehling C, Kelley DA, Majumdar S, Link TM (2009) Imaging of the musculoskeletal system in vivo using ultra-high field magnetic resonance at 7 T. Invest Radiol 44:613–618
Hoult DI, Phil D (2000) Sensitivity and power deposition in a high-field imaging experiment. J Magn Reson Imaging 12:46–67
Mao W, Smith MB, Collins CM (2006) Exploring the limits of RF shimming for high-field MRI of the human head. Magn Reson Med 56:918–922
Van de Moortele PF, Akgun C, Adriany G et al (2005) B(1) destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med 54:1503–1518
Orzada S, Quick HH, Ladd ME et al (2009) A flexible 8-channel transmit/receive body coil for 7 T human imaging. 17th Scientific Meeting ISMRM, Hawaii, USA, pp 2999
Ellermann J, Goerke U, Morgan P et al (2012) Simultaneous bilateral hip joint imaging at 7 Tesla using fast transmit B(1) shimming methods and multichannel transmission - a feasibility study. NMR Biomed 25:1202–1208
Chang G, Deniz CM, Honig S et al (2014) MRI of the hip at 7T: feasibility of bone microarchitecture, high-resolution cartilage, and clinical imaging. J Magn Reson Imaging 39:1384–1393
Theysohn JM, Kraff O, Orzada S et al (2013) Bilateral hip imaging at 7 Tesla using a multi-channel transmit technology: initial results presenting anatomical detail in healthy volunteers and pathological changes in patients with avascular necrosis of the femoral head. Skeletal Radiol 42:1555–1563
Theysohn JM, Kraff O, Theysohn N et al (2014) Hip imaging of avascular necrosis at 7 Tesla compared with 3 Tesla. Skeletal Radiol 43:623–632
Welsch GH, Mamisch TC, Hughes T et al (2008) In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest Radiol 43:619–626
Pakin SK, Cavalcanti C, La Rocca R, Schweitzer ME, Regatte RR (2006) Ultra-high-field MRI of knee joint at 7.0T: preliminary experience. Acad Radiol 13:1135–1142
Welsch GH, Apprich S, Zbyn S et al (2011) Biochemical (T2, T2* and magnetisation transfer ratio) MRI of knee cartilage: feasibility at ultra-high field (7T) compared with high field (3T) strength. Eur Radiol 21:1136–1143
Chang G, Xia D, Sherman O et al (2013) High resolution morphologic imaging and T2 mapping of cartilage at 7 Tesla: comparison of cartilage repair patients and healthy controls. MAGMA 26:539–548
Nehrke K, Bornert P (2012) DREAM–a novel approach for robust, ultrafast, multislice B(1) mapping. Magn Reson Med 68:1517–1526
Kraff O, Lazik A, Brenner D et al (2015) In vivo comparison of B1 mapping techniques for hip joint imaging at 7 Tesla. 23rd Scientific Meeting ISMRM, Toronto, Canada
Manuel A, Li W, Jellus V, Hughes T, Prasad PV (2011) Variable flip angle-based fast three-dimensional T1 mapping for delayed gadolinium-enhanced MRI of cartilage of the knee: need for B1 correction. Magn Reson Med 65:1377–1383
Siversson C, Chan J, Tiderius CJ et al (2012) Effects of B1 inhomogeneity correction for three-dimensional variable flip angle T1 measurements in hip dGEMRIC at 3 T and 1.5 T. Magn Reson Med 67:1776–1781
Deoni SC, Rutt BK, Peters TM (2003) Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med 49:515–526
Fram EK, Herfkens RJ, Johnson GA et al (1987) Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging 5:201–208
Noel P, Bammer R, Reinhold C, Haider MA (2009) Parallel imaging artifacts in body magnetic resonance imaging. Can Assoc Radiol J 60:91–98
Lazik A, Korsmeier K, Classen T et al (2014) 3 Tesla high-resolution and delayed gadolinium enhanced MR imaging of cartilage (dGEMRIC) after autologous chondrocyte transplantation in the hip. J Magn Reson Imaging. doi:10.1002/jmri.24821
Siversson C, Akhondi-Asl A, Bixby S, Kim YJ, Warfield SK (2014) Three-dimensional hip cartilage quality assessment of morphology and dGEMRIC by planar maps and automated segmentation. Osteoarthritis Cartilage 22:1511–1515
Bittersohl B, Hosalkar HS, Kim YJ, Werlen S, Siebenrock KA, Mamisch TC (2009) Delayed gadolinium-enhanced magnetic resonance imaging (dGEMRIC) of hip joint cartilage in femoroacetabular impingement (FAI): are pre- and postcontrast imaging both necessary? Magn Reson Med 62:1362–1367
Williams A, Mikulis B, Krishnan N, Gray M, McKenzie C, Burstein D (2007) Suitability of T(1Gd) as the dGEMRIC index at 1.5T and 3.0T. Magn Reson Med 58:830–834
Rooney WD, Johnson G, Li X et al (2007) Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med 57:308–318
Zilkens C, Miese F, Kim YJ et al (2012) Three-dimensional delayed gadolinium-enhanced magnetic resonance imaging of hip joint cartilage at 3T: a prospective controlled study. Eur J Radiol 81:3420–3425
Bittersohl B, Hosalkar HS, Haamberg T et al (2009) Reproducibility of dGEMRIC in assessment of hip joint cartilage: a prospective study. J Magn Reson Imaging 30:224–228
Bittersohl B, Miese FR, Hosalkar HS et al (2012) T2* mapping of acetabular and femoral hip joint cartilage at 3 T: a prospective controlled study. Invest Radiol 47:392–397
Lattanzi R, Petchprapa C, Glaser C et al (2012) A new method to analyze dGEMRIC measurements in femoroacetabular impingement: preliminary validation against arthroscopic findings. Osteoarthritis Cartilage 20:1127–1133
Trattnig S, Ohel K, Mlynarik V, Juras V, Zbyn S, Korner A (2015) Morphological and compositional monitoring of a new cell-free cartilage repair hydrogel technology - GelrinC by MR using semi-quantitative MOCART scoring and quantitative T2 index and new zonal T2 index calculation. Osteoarthritis Cartilage. doi:10.1016/j.joca.2015.07.007
Bittersohl B, Hosalkar HS, Werlen S, Trattnig S, Siebenrock KA, Mamisch TC (2011) dGEMRIC and subsequent T1 mapping of the hip at 1.5 Tesla: normative data on zonal and radial distribution in asymptomatic volunteers. J Magn Reson Imaging 34:101–106
Yoshida K, Azuma H (1982) Contents and compositions of glycosaminoglycans in different sites of the human hip joint cartilage. Ann Rheum Dis 41:512–519
Watanabe A, Boesch C, Siebenrock K, Obata T, Anderson SE (2007) T2 mapping of hip articular cartilage in healthy volunteers at 3T: a study of topographic variation. J Magn Reson Imaging 26:165–171
Acknowledgments
The authors thank Desmond Tse (Maastricht University, The Netherlands) for providing the source code of the DREAM sequence.
This work was supported by a research grant of the University Duisburg-Essen, Germany, awarded to the first author.
The scientific guarantor of this publication is Dr. med. Andrea Lazik. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. This study has received funding from the University of Duisburg-Essen: A research grant (“IFORES”) was awarded to the first author. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazik, A., Theysohn, J.M., Geis, C. et al. 7 Tesla quantitative hip MRI: T1, T2 and T2* mapping of hip cartilage in healthy volunteers. Eur Radiol 26, 1245–1253 (2016). https://doi.org/10.1007/s00330-015-3964-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3964-0