Abstract
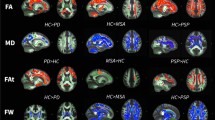
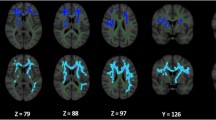
Diffusion tensor imaging (DTI) appears as a sensitive method to study Parkinson’s disease (PD) pathophysiology and severity. Fractional anisotropy (FA) value is one of the scalar derivatives of DTI used to find out anisotropy within a voxel in a tissue and used for determining white matter integrity in aging and neurodegenerative diseases. We studied DTI derived FA in early PD subjects as their routine MRI scans were normal. 40 patients with early PD and 40 healthy controls were employed to evaluate changes in microstructural white and grey matter in the brain’s using DTI derived FA values. Comparison of FA values in the brain’s white and grey matter of patients with PD and age matched controls at the corpus callosum, centrum semiovale, pons, putamen, caudate nucleus, substantia nigra, cerebral peduncles and cerebellar peduncles, was done using a region of interest (ROI) technique, with b-value 1000s/mm2 and TE = 100 milliseconds using 1.5 T MRI system. PD patients showed differences in FA values in both the grey and white matter areas of the brain’s compared to healthy controls. Our study revealed the presence of damage in the substantia nigra, corpus callosum, putamen and cerebral peduncles mainly in the PD group. Our findings indicate that DTI and region of interest (ROI) methods can be used in patients with early PD to study microstructural alterations mainly in the substantia nigra, putamen and corpus callosum.






Similar content being viewed by others
Data availability
The data collected in the current study comprises of screenshot images taken after obtaining FA values of PD patients and healthy controls using diffusion tensor imaging fibre track software. The data will be shared on request as per the Kasturba Medical hospital ethical committee patient privacy guidelines. The datasets used and/or analysed during the current study is available from the corresponding author on reasonable request.
References
Daniel SE, Lees AJ. Parkinson's disease society brain Bank, London: overview and research. Journal of neural transmission Supplementum. 1993;39:165.
Gattellaro G, Minati L, Grisoli M, Mariani C, Carella F, Osio M, et al. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. Am J Neuroradiol. 2009 Jun 1;30(6):1222–6.
Skidmore FM, Yang M, Baxter L, Von Deneen KM, Collingwood J, He G, et al. Reliability analysis of the resting state can sensitively and specifically identify the presence of Parkinson disease. Neuroimage. 2013 Jul 15;75:249–61.
Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006 Sep 7;51(5):527–39.
Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007 Jul 1;4(3):316–29.
Zhang K, Yu C, Zhang Y, Wu X, Zhu C. Voxel-based analysis of diffusion tensor indices in the brain in patients with Parkinson ’ s disease. Eur J Radiol [Internet]. 2017;77(2):269–73.
Saeed U, Compagnone J, Aviv RI, Strafella AP, Black SE, Lang AE, et al. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: current and emerging concepts. Translational neurodegeneration. 2017 Dec;6(1):8.
Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013 Feb 26;80(9):857–64.
Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: a region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage: Clinical. 2013 Jan 1;3:481–8.
Atkinson-Clement C, Pinto S, Eusebio A, Coulon O. Diffusion tensor imaging in Parkinson's disease: review and meta-analysis. NeuroImage: Clinical. 2017 Jan 1;16:98–110.
Chan LL, Ng KM, Yeoh CS, Rumpel H, Li HH, Tan EK. Putaminal diffusivity correlates with disease progression in parkinson's disease: prospective 6-year study. Medicine. 2016 Feb;95(6).
Zhan W, Kang GA, Glass GA, Zhang Y, Shirley C, Millin R, et al. Regional alterations of brain microstructure in Parkinson’s disease using diffusion tensor imaging. Mov Disord. 2012;27(1):90–7.
Langley J, Huddleston DE, Merritt M, Chen X, McMurray R, Silver M, et al. Diffusion tensor imaging of the substantia nigra in Parkinson’s disease revisited. Hum Brain Mapp. 2016;37(7):2547–56.
Duncan GW, Firbank MJ, Yarnall AJ, Khoo TK, Brooks DJ, Barker RA, et al. Gray and white matter imaging: a biomarker for cognitive impairment in early Parkinson’s disease? Mov Disord. 2016;31(1):103–10.
Chen N-K, Chou Y, Sundman M, Hickey P, Kasoff WS, Bernstein A, et al. Alteration of diffusion-tensor MRI measures in brain regions involved in early stages of Parkinson’s disease. Brain Connect. 2018;brain.2017.0558.
Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, et al. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol. 2011;258(7):1254–60.
Joshi N, Rolheiser TM, Fisk JD, McKelvey JR, Schoffer K, Phillips G, et al. Lateralized microstructural changes in early-stage Parkinson’s disease in anterior olfactory structures, but not in substantia nigra. J Neurol. 2017;264(7):1497–505.
Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: a region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage Clin. 2013;3:481–8.
Melzer TR, Watts R, Macaskill MR, Pitcher TL, Livingston L, Keenan RJ, et al. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology. 2013;80(20):1841–9.
Pozorski V, Oh JM, Adluru N, Merluzzi AP, Theisen F. Okonkwo O, et al. Longitudinal white matter microstructural change in Parkinson’s disease. 2018:1–12.
Prakash BD, Sitoh Y-Y, Tan LCS, Au WL. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson’s disease. Parkinsonism Relat Disord. 2012 Nov;18(9):1029–33.
Zhang Y, Wu IW, Tosun D, Foster E, Schuff N. Progression of regional microstructural degeneration in Parkinson’s disease: a multicenter diffusion tensor imaging study. PLoS One. 2016;11(10):1–16.
Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, et al. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009 Apr 21;72(16):1378–84.
Péran P, Cherubini A, Assogna F, Piras F, Quattrocchi C, Peppe A, et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010 Nov;133(11):3423–33.
Chan L-L, Rumpel H, Yap K, Lee E, Loo H-V, Ho G-L, et al. Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007 Dec 1;78(12):1383–6.
Chan LL, Ng KM, Yeoh CS, Rumpel H, Li HH, Tan EK. Putaminal diffusivity correlates with disease progression in Parkinson’s disease. Med (United States). 2016;95(6):1–4.
Rulseh AM, Keller J, Tintěra J, Kožíšek M, Vymazal J. Chasing shadows: what determines DTI metrics in gray matter regions? An in vitro and in vivo study. J Magn Reson Imaging. 2013 Nov;38(5):1103–10.
Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol Aging. 2010 Mar;31(3):482–93.
White ML, Zhang Y. Three-tesla diffusion tensor imaging of Meyer’s loop by tractography, color-coded fractional anisotropy maps, and eigenvectors. Clin Imaging. 2010 Jan;34(6):413–7.
Goldman JG, Bledsoe IO, Merkitch D, Dinh V, Bernard B, Stebbins GT. Corpus callosal atrophy and associations with cognitive impairment in Parkinson disease. Neurology. 2017 Mar 28;88(13):1265–72.
Wiltshire K, Foster S, Kaye JA, Small BJ, Camicioli R. Corpus callosum in neurodegenerative diseases: findings in Parkinson’s disease. Dement Geriatr Cogn Disord. 2005;20(6):345–51.
Meijer FJA, Van Rumund A, Tuladhar AM. Conventional 3T brain MRI and diffusion tensor imaging in the diagnostic workup of early stage parkinsonism. Neuroradiology. 2015;57:655–69.
Meijer FJA, Bloem BR, Mahlknecht P, Seppi K, Goraj B. Update on diffusion MRI in Parkinson’s disease and atypical parkinsonism. J Neurol Sci. 2013;332(1–2):21–9.
Funding
Bombay Scientific, Mumbai, Maharashtra, India.
Author information
Authors and Affiliations
Contributions
RK conceptualized the study. RK, PK and SN have given inputs in study design. RK collected the data. RK analysed the data and wrote the first draft of manuscript and all co- authors contributed in critical review of data analysis and manuscript writing. RK will act as guarantor for this paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests in this study.
Ethical approval
The study protocol followed was reviewed and approved by the Research Committee of Manipal College of Health Profession and Manipal Academy of Higher Education, and ethical clearance was also obtained by Kasturba Medical College and Hospital, MAHE, Manipal.
Informed consent
A detailed explanation about the study was given by the principal investigator after which they provided consent for publication. All the patients included in this research gave written informed consent to publish the data contained within this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kotian, R.P., Prakashini, K. & Nair, N.S. A diffusion tensor imaging study to compare normative fractional anisotropy values with patients suffering from Parkinson’s disease in the brain grey and white matter. Health Technol. 10, 1283–1289 (2020). https://doi.org/10.1007/s12553-020-00454-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12553-020-00454-1