Abstract
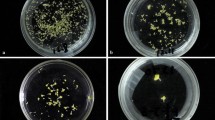
An efficient in vitro mutagenesis protocol for Lilium longiflorum Thunb. cv. White fox has been established. The effect of 6-BA and NAA on adventitious bud formation from the bulblet-scale thin cell layers was tested. Results showed that the optimal medium for adventitious bud induction is MS basal medium supplemented with 2.0 mg/l 6-BA and 0.1 mg/l NAA. The differentiation frequency and the average number of adventitious buds reached 95.55% and 3.00, respectively. Various doses (0.0, 0.5, 1.0, 1.5, 2.0, and 2.5 Gy) of gamma rays were applied to investigate the effect of radiation on adventitious bud formation from bulblet-scale thin cell layers. The forming capacity of the adventitious buds significantly decreased with the increase of radiation dose. The results suggested that the optimal irradiation dose is 1.0 Gy. Dose of 1.0 Gy treatment resulted in 55.33% survival of irradiated bulblet-scale thin cell layers and 39.27% mutagenesis rate. The genetic variations among the morphological mutants were evaluated by DNA fingerprinting using ISSR molecular marker. The genetic variation frequency reached 36.06% using seven ISSR primers. Out of the 50 mutant lines transferred to the greenhouse, 9 were observed to have significantly different morphological characters than those of the controls.



Similar content being viewed by others
Abbreviations
- 6-BA:
-
6-Benzylaminopurine
- CTAB:
-
Hexadecyltrimethyl ammonium bromide
- ISSR:
-
Inter simple sequence repeat
- MS:
-
Murashige and Skoog (1962)
- NAA:
-
a-Naphthaleneacetic acid
- TCL:
-
Thin cell layers
References
Abe H, Nakano M, Nakatsuka A, Nakayama M, Koshioka M, Yamagishi M (2002) Genetic analysis of floral anthocyanin pigmentation traits in Asiatic hybrid lily using molecular linkage maps. Theor Appl Genet 105:1175–1182. doi:10.1007/s00122-002-1053-7
Alikamanoglu S (2002) Efficiency of the gamma irradiation in the induction of in vitro somatic mutations. J Cell Mol Biol 1:19–24
Al-Safadi B, Ayyoubi Z, Jawdat D (2000) The effect of gamma irradiation on potato microtuber production in vitro. Plant Cell Tissue Organ Cult 61:183–187. doi:10.1023/A:1006477224536
Arunyanart S, Soontronyatara S (2002) Mutation induction by γ and X-ray irradiation in tissue cultured lotus. Plant Cell Tissue Organ Cult 70:119–122. doi:10.10023/A:1016021627832
Barakat MN, Abdel Fattah RS, Badr M, El-Torky MG (2010) In vitro mutagenesis and identification of new variants via RAPD markers for improving Chrysanthemum morifolium. Afr J Agric Res 5:748–757
Bhagwat B, Duncan EJ (1998) Mutation breeding of highgate (Musa acuminata, AAA) for tolerance to Fusarium oxysporum f. sp. cubense using gamma irradiation. Euphytica 101:143–150. doi:10.1023/A1018391619986
Das A, Gosal SS, Sidhu JS, Dhaliwal HS (2000) Induction of mutations for heat tolerance in potato by using in vitro culture and radiation. Euphytica 114:205–209. doi:10.1023/A:1003965724880
Devarumath RM, Nandy S, Rani V, Marimuthu S, Muraleedharan N, Raina SN (2002) RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type). Plant Cell Rep 21:166–173. doi:10.1007/s00299-002-0496-2
Han DS, Niimi Y, Nakano M (1997) Regeneration of haploid plants from anther cultures of the Asiatic hybrid lily ‘connecticut king’. Plant Cell Tissue Organ Cult 47:153–158. doi:10.1007/BF02318951
Hung CD, Johnson K (2008) Effects of ionizing radiation on the growth and allyl isothiocyanate accumulation of Wasabia japonica in vitro and ex vitro. In Vitro Cell Dev Biol Plant 44:51–58. doi:10.1007/S11627-007-9083-0
Jain SM (2002) A review of induction of mutations in fruits of tropical and subtropical regions. Acta Hortic 575:295–302
Joseph R, Yeoh HH, Loh CS (2004) Induced mutations in cassava using somatic embryos and the identification of mutant plants with altered starch yield and composition. Plant Cell Rep 23:91–98. doi:10.1007/S0029-004-0798-7
Kato Y, Yasutake Y (1977) Plantlet formation and differentiation of epidermal tissue in green callus cultures from excised leaves of Lilium. Phytomorphology 27:390–396
Kazama Y, Hirano T, Saito H, Liu Y, Ohbu S, Hayashi Y, Abe T (2011) Characterization of highly efficient heavy-ion mutagenesis in Arabidopsis thaliana. BMC Plant Biol 11:161. doi:10.1186/1471-2229-11-161
Lee YI, Lee IS, Lim YP (2002) Variations in sweetpotato regenerated from gamma-ray irradiated embryogenic callus. J Plant Biotechnol 4:163–170
Lee SY, Cheong JI, Kim TS (2003) Production of doubled haploids through anther culture of M1 rice plants derived from mutagenized fertilized egg cells. Plant Cell Rep 22:218–223. doi:10.1007/S00299-003-0663-0
Leslie AC (1982–2005) The international lily register (including supplements). The Royal Horticultural Society, London
Lu G, Zhang XY, Zou YJ, Zou QC, Xiang X, Cao JS (2007) Effect of radiation on regeneration of Chinese narcissus and analysis of genetic variation with AFLP and RAPD markers. Plant Cell Tissue Organ Cult 88:319–327. doi:10.1007/S11240-006-9189-9
Muller HJ (1927) Artificial transmutation of the gene. Science 66:84–87. doi:10.1126/science.66.1699.84
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nhut DT (1998) Micropropagation of lily (Lilium longiflorum) via in vitro stem node and pseudo-bulblet culture. Plant Cell Rep 17:913–916. doi:10.1007/S002990050508
Nhut DT, Bui VL, Fukai S, Tanaka M, Tran Thanh Van K (2001a) Effects of activated charcoal, explant size, explant position and sucrose concentration on plant and shoot regeneration of Lilium longiflorum via young stem culture. Plant Growth Regul 33:59–65. doi:10.1023/A:1010701024963
Nhut DT, Bui VL, Silva DA, Aswath CR (2001b) The cell layer culture system in Lilium:regeneration and transformation perspectives. In Vitro Cell Dev Biol Plant 37:516–523. doi:10.1007/S11627-001-009-2
Nhut DT, Bui VL, Tanaka M, Tran Thanh Van K (2001c) Shoot induction and plant regeneration from receptacle tissue of Lilium longiflorum. Sci Hortic 87:131–138. doi:10.1016/S0304-4238(00)00163-1
Nhut DT, Bui VL, Tran Thanh Van K (2001d) Manipulation of the morphogenetic pathways of Lilium longiflorum transverse thin cell layer explants by auxin and cytokinin. In Vitro Cell Dev Biol Plant 37:44–49. doi:10.1007/S11627-001-0009-y
Nhut DT, Bui VL, Minh NT, Teixeira da Silva JA, Fukai S, Tanaka M, Tran Thanh Van K (2002) Somatic embryogenesis through pseudo-bulblet transverse thin cell layer of Lilium longiflorum. Plant Growth Regul 37:193–198. doi:10.1023/A:1020532511081
Nhut DT, Hanh NTM, Tuan PQ, Nguyet LTM, Tram NTH, Chinh NC, Nguyen NH, Vinh DN (2006) Liquid culture as a positive condition to induce and enhance quality and quantity of somatic embryogenesis of Lilium longiflorum. Sci Hortic 110:93–97. doi:10.1016/j.scienta.2006.05.015
Puchooa D (2005) In vitro mutation breeding of anthurium by gamma radiation. Int J Agric Biol 7:11–20
Simmonds JA, Cumming BG (1976a) Propagation of Lilium hybrids. I. Dependence of bulblet production on time of scale removal and growth substances. Sci Hortic 5:77–83. doi:10.1016/03044238(76)90026-1
Simmonds JA, Cumming BG (1976b) Propagation of Lilium hybrids. II. Production of plantlets from bulb-scale callus cultures for increased propagation. Sci Hortic 5:161–170. doi:10.1016/030442338(76)90078-9
Stadler LJ (1928) Mutations in barley induced by X-rays and radium. Science 68:186–187. doi:10.1126/science.68.1756.186
Stanilova MI, Ilcheva VP, Zagorska NA (1994) Morphogenetic potential and in vitro micropropagation of endangered plant species Leucojum aestivum L. and Lilium rhodopaeum Delip. Plant Cell Rep 13:451–453. doi:10.1007/BF00231965
Teixeira da Silva JA (2003) Thin Cell Layer technology in ornamental plant micropropagation and biotechnology. Afr J Biotechnol 2:683–691
Tribulato A, Remotti PC, Loffler HJM, Van Tuyl JM (1997) Somatic embryogenesis and plant regeneration in Lilium longiflorum Thumb. Plant Cell Rep 17:113–118. doi:10.1007/S002990050362
Varshney A, Lakshmikumaran M, Srivastava PS, Dhawan V (2001) Establishment of genetic fidelity of in vitro-raised Lilium bulblets through RAPD markers. In Vitro Cell Dev Biol Plant 37:227–231. doi:10.1079/IVP2000132
Velmurugan M, Rajamani K, Paramaguru P, Gnanam R, Bapu JR Kannan, Harisudan C, Hemalatha P (2010) In vitro mutation in horticultural crops––a review. Agric Rev 31:63–67
Wu XP, Shi JS, Xi ML, Luo ZW, Hu XH (2010) A B functional gene cloned from lily encodes an ortholog of Arabidopsis PISTILLATA (PI). Plant Mol Biol Rep 28:684–691. doi:10.1007/S11105-010-0193-1
Yamagishi M (2003) A genetic model for a pollenless trait in Asiatic hybrid lily and its utilization for breeding. Sci Hortic 98:293–297. doi:10.1016/S03044238(02)00230-3
Yamaguchi H, Nagatomi S, Morishita T, Degi K, Tanaka A, Shikazono N, Hase Y (2003) Mutation induced with ion beam irradiation in rose. Nucl Instrum Methods Phys Res B 206:561–564. doi:10.1016/S0168-583x(03)00825-5
Zhang KZ, Zhao XY, Huang SW, Lu CX, Liang L (2002) Effects of radiation on M1 plants and scale cutting’s initiating adventitious bud plants. J Bejing Agri Coll 17:19–25
Acknowledgments
The authors wish to thank Professor Zhongen Xi (Chongqing University of Posts and Telecommunications, China) for his valuable assistance in preparation of the manuscript. Financial support from the National Natural Science Foundation of China (30972407), Jiangsu Provincial Key Basic Research foundation for Universities (07KJA21019), “948” project of the State Administration of Forestry of China (2007-4-06), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Schmidt.
Rights and permissions
About this article
Cite this article
Xi, M., Sun, L., Qiu, S. et al. In vitro mutagenesis and identification of mutants via ISSR in lily (Lilium longiflorum). Plant Cell Rep 31, 1043–1051 (2012). https://doi.org/10.1007/s00299-011-1222-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1222-8