Abstract
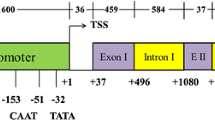
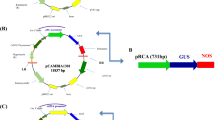
The rice Ubiquitin1 (Ubi1) promoter was tested to evaluate its capacity to express the heterologous gusA gene encoding β-glucuronidase in transgenic rice tissue relative to the commonly used Ubi1 corn promoter and the rice gibberellic acid insensitive (GAI) gene promoter element. Experimental results showed increased expression of gusA gene in rice tissue when driven by the native Ubi1 promoter when compared to the use of corn Ubi1 promoter. Results further indicated that the cis-regulatory elements present in the native promoter element might have been responsible for high expression. However, the gusA gene expression level when driven by the rice GAI promoter was notably lower than both Ubi1 promoters. The present study, thus, for the first time helped to demonstrate that the native Ubi1 promoter is a promising genetic element in transgenic approaches for constitutive expression of any gene in rice tissue.





Similar content being viewed by others
References
An YQ, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB (1996) Strong, constructive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J 10:107–121
Baszcynski CL, Barbour E, Miki B (1997) ALS3 promoters. US Patent 5659026
Bhattacharyya J (2010) Transfer of alien entomocidal gene(s) in a commercial rice cultivar of India. Ph.D. Dissertation, Calcutta University, India
Binet MN, Lepetit M, Weil JH, Tessier LH (1991a) Analysis of a sunflower polyubiquitin promoter by transient expression. Plant Sci 79:87–94
Binet MN, Weil JH, Tesseir LH (1991b) Structure and expression of sunflower ubiquitin genes. Plant Mol Biol 17:395–407
Bradford MM (1976) A rapid and sensitive method for the qualification of microgram quantities of protein utilizing the principle-dye binding. Anal Biochem 72:248–254
Burke TJ, Callis J, Vierstra RD (1988) Characterization of a polyubiquitin gene from Arabidopsis thaliana. Mol Gen Genet 213:435–443
Callis J, Raasch JA, Vierstra RD (1990) Ubiquitin extension proteins of Arabidopsis thaliana-structure, localization, and expression of their promoters in transgenic tobacco. J Biol Chem 265:12486–12493
Cazzonelli CI, McCallum EJ, Lee R, Botella JR (2005) Characterization of a strong constitutive mung bean (Vigna radiata L.). Promoters with a complex mode of regulation in planta. Transgenic Res 14:941–967
Chiera JM, Bouchard RA, Dorsey SL, Park EH, Buenrostro-Nava MT, Ling PP, Finer JJ (2007) Isolation of two highly active soybean (Glycine max (L.) Merr.) promoters and their characterization using a new automated image collection and analysis system. Plant Cell Rep 26:1501–1509
Chowdhury AH (2005) Development of transgenic rice plants with altered growth pattern. Ph.D. dissertation, Jadavpur University, Calcutta, India
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high levels of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Christensen AH, Sharrok RA, Quail PH (1992) Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18:675–689
Cornejo MJ, Luth D, Blankenship KM, Anderson OD, Blechl A (1993) Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol Biol 23:567–581
DeBuck S, Depicker A (2001) Disruption of their palindromic arrangement leads to selective loss of DNA methylation in inversely repeated gus transgenes in Arabidopsis. Mol Genet Genom 265:1060–1068
DeBuck S, Depicker A (2004) Gene expression and level of expression. In: Christou P, Klee H (eds) Handbook of plant biotechnology. John Wiley, Chichester, pp 331–345
DeBuck S, VanMontagu M, Depicker A (2001) Transgene silencing of invertedly repeated transgenes is released upon deletion of one of the transgenes involved. Plant Mol Biol 46:433–445
Doyle JJ, Doyle JJ (1990) Isolation of plant DNA form fresh tissue. Focus 12:13–15
Furtado A, Henry RJ, Takaiwa F (2008) Comparison of promoters in transgenic rice. Plant Biotech J 6:679–693
Garbarino JE, Oosumi T, Belknap WR (1995) Isolation of a polyubiquitin promoter and its expression in transgenic potato plants. Plant Physiol 109:1371–1378
Genschik P, Marbach J, Uze M, Feuerman M, Plesse B, Fleck J (1994) Structure and promoter activity of a stress and developmentally regulated polyubiquitin-encoding gene of Nicotiana tabacum. Gene 148:195–202
He C, Lin Z, McElroy D, Wu R (2009) Identification of a rice actin2 gene regulatory region for high-level expression of transgenes in monocots. Plant Biotechnol J 7:227–239
Hermann SR, Harding RM, Dale JL (2001) The banana actin1 promoter drives near-constitutive transgene expression in vegetative tissues of banana (Musa spp.). Plant Cell Rep 20:525–530
Hernandez-Garcia CM, Martinelli AP, Bouchard RA, Finer JJ (2009) A soybean (Glycine max) polyubiquitin promoter gives strong constitutive expression in transgenic soybean. Plant Cell Rep 28:837–849
Hiei Y, Komari T (2008) Agrobacterium mediated transformats of rice using immature embryos or calli induced from mature seed. Nat Protoc 3(5):824–834
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acid Res 27(1):297–300
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene-transfer to plants. Transgenic Res 2:208–218
Itoh H, Veguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellins signaling path way is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14:57–70
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Joung YH, Kamo K (2006) Expression of a polyubiquitin promoter isolated from Gladiolus. Plant Cell Rep 25:1081–1088
Kamo K, Blowers A, Smith F, Van Eck J, Lawson R (1995) Stable transformation of Gladiolus using suspension cells and callus. J Am Soc Hortic Sci 120:347–352
Kawalleck PP, Somssich IE, Feldbrugge M, Hahlbrock K, Weisshaar B (1993) Polyubiquitin gene expression and structural properties of the ubi4-2 gene in Petroselium crispum. Plant Mol Biol 21:673–684
Lescot M et al (2002) PlantCARE, a database of plant cis-acting regulatory elements a portal to tools for in silico analysis of promoter sequences. Nucleic Acid Res 30(1):325–327
Mandel T, Fleming AJ, Krahenbuhl R, Kuhlemeier C (1995) Definition of constitutive gene expression in plants: the translation initiation factor 4A gene as a model. Plant Mol Biol 29:995–1004
McElroy D, Zhang W, Wu R (1990) Isolation of an efficient actin promoter for use in rice transformation. Plant Cell 2:163–171
McElroy D, Blowers A, Jenes B, Wu R (1991) Construction of expression vectors based on the rice actin1 (Act1) 5′ region for use in monocot transformation. Mol Gen Genet 231:150–160
O’Connor TR, Dyreson C, Wyrick JJ (2005) Athena: a resource for rapid visualization and systematic analysis of Arabidopsis promoter sequences. Bioinformatics 21(24):441–4413
Potenza C, Aleman L, Sengupta-Gopalan C (2004) Targeting transgene expression in research, agricultural, and environmental applications: promoters used in plant transformation. In Vitro Cell Dev Biol Plant 40:1–22
Prestridge DS (1995) Predicting polII promoter sequences using transcription factor binding sites. J Mol Biol 249(5):923–932
Rooke L, Byrne D, Salgueiro S (2000) Marker gene expression driven by the maize ubiquitin promoters in transgenic wheat. Ann App Biol 136:167–172
Rose AB, Beliakoff JA (2000) Intron-mediated enhancement of gene expression independent of unique intron sequences and splicing. Plant Physiol 22:535–542
Rushton PJ, Reinstädler A, Lipka V, Lippok B, Somssich IE (2002) Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen- and wound-induced signaling. Plant Cell 14:749–762
Sambrook J, Fritsch EF, Maniatist T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold spring Harbor Laboratory press, Plainview
Shahmuradov IA, Solovyev VV, Gammerman AJ (2005) Plant promoter prediction with confidence estimation. Nucleic Acid Res 33(3):1069–1076
Shivamani E, Qu R (2006) Expression enhancement of a rice polyubiquitin gene promoter. Plant Mol Biol 60:225–239
Sullivan JA, Shirasu K, Deng XW (2003) The diverse roles of ubiquitin and the 26S proteasome in the life of plants. Nat Rev Genet 4:948–958
Tiana L, Wub K, Hannama C, Latoszek-Greena M, Sibbalda S, Huc M, Browna DCW, Mikic B (2005) Analysis and use of the tobacco elF4A-10 promoter elements for transgene expression. J Plant Physiol 162:1335–1366
Twyman RM (2003) Growth and development: control of gene expression, regulation of transcription. In: Thomas B, Murphy DJ, Murray B (eds) Encyclopedia of applied plant sciences. Elesvier, London, pp 558–567
Wang JL, Jiang JD, Oard JH (2000) Structure expression and promoter activity of two polyubiquitin genes from rice (Oryza sativa L.). Plant Sci 156:201–211
Wei HR, Wang ML, More PH, Albert HH (2003) Comparative expression analysis of two sugarcane polyubiquitin promoters and flanking sequences in transgenic plants. J Plant Physiol 160:1241–1251
Acknowledgments
Authors register their appreciation for the technical help received from Sona Dogra, Partho Das and Meghnath Prasad. Authors are also thankful to the two anonymous reviewers whose critical comments and helpful suggestions helped in improving the clarity of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
Rights and permissions
About this article
Cite this article
Bhattacharyya, J., Chowdhury, A.H., Ray, S. et al. Native polyubiquitin promoter of rice provides increased constitutive expression in stable transgenic rice plants. Plant Cell Rep 31, 271–279 (2012). https://doi.org/10.1007/s00299-011-1161-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1161-4