Abstract
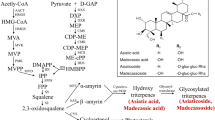
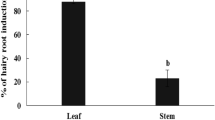
Transformed root (“hairy root”) cultures have been shown to be a good model for the study of many secondary metabolites. However, economically important compounds such as asiaticoside and madecassoside are produced in insignificant amounts in the root of Centella asiatica (L.) Urban. To overcome this problem, C. asiatica was transformed using Agrobacterium rhizogenes strain R1000 that harbors pCAMBIA1302 encoding the hygromycin phosphotransferase (hpt) and green fluorescence protein (mgfp5) genes and the hairy culture was coupled with elicitation technique. Hairy roots were obtained at a frequency of up to 14.1% from a tissue junction between the leaf and petiole. Abundant hairy roots were observed when co-cultivation of the plant with A. rhizogenes was done for 7 days (36.1%). Transformation was confirmed by PCR and Southern blot analyses. Five weeks after inoculation, no asiaticoside was detected in the hairy root samples. However, when 0.1 mM methyl jasmonate (MJ) was applied as an elicitor to the culture medium for 3 weeks, a large quantity of asiaticoside was generated (7.12 mg/g, dry wt). In the case of gene expression, 12 h after MJ treatment the expression of the CabAS (C. asiatica putative β-amyrin synthase) gene in the hairy roots is significantly different from that of the control and this level of transcripts was maintained for 14 days. Our results showed that production of C. asiatica hairy roots could be optimized and the resulting cultures could be elicited with MJ treatment for enhanced production of asiaticoside.






Similar content being viewed by others
Abbreviations
- CabAS :
-
Centella asiatica putative beta-amyrin synthase
- GFP:
-
Green fluorescence protein
- HPLC:
-
High-performance liquid chromatography
- hpt :
-
Hygromycin phosphotransferase
- MJ:
-
Methyl jasmonate
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
References
Alpizar E, Dechamp E, Espeout S, Royer M, Lecouls AC, Nicole M, Bertrand B, Lashermes P, Etienne H (2006) Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants for studying gene expression in coffee roots. Plant Cell Rep 25:959–967
Aoyagi H, Kobayashi Y, Yamada K, Yokoyama K, Kusakari K, Tanaka H (2001) Efficient production of saikosaponins in Bupleurum falcatum root fragments combined with signal transducers. Appl Microbiol Biotechnol 57:482–488
Aziz ZA, Davey MR, Power JB, Anthony P, Smith RM, Lowe KC (2007) Production of asiaticoside and madecassoside in Centella asiatica in vitro and in vivo. Biol Plant 51:34–42
Baek YW (1997) Micropropagation of Centella asiatica (L.) Urban by in vitro cultures and production of triterpene glycosides. PhD thesis, Chonnam University, Gwangju
Bercetche J, Chriqui D, Adam S, David C (1987) Morphogenetic and cellular reorientation induced by Agrobacterium rhizogenes (strains 1855, 2659 and 8196) on carrot, pea and tobacco. Plant Sci 52:195–210
Bonfill M, Mangas S, Cusidó RM, Osuna L, Piñol MT, Palazón J (2006) Identification of triterpenoid compounds of Centella asiatica by thin-layer chromatography and mass spectrometry. Biomed Chromatogr 20:151–153
Chen DT, Ye HC, Li GF (2000) Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Damgaard O, Rasmussen O (1991) Direct regeneration of transformed shoots in Brassica napus from hypocotyls infections with Agrobacterium rhizogenes. Plant Mol Biol 17:1–8
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Flores HE, Vivanco JM, Loyola-Vargas VM (1999) Radicle biochemistry: the biology of root-specific metabolism. Trends Plant Sci 4:220–226
Hamill JD, Rounsley S, Spencer A, Todd G, Rhodes MJC (1991) The use of the polymerase chain reaction in plant transformation studies. Plant Cell Rep 10:221–224
Hayashi H, Huang PY, Inoue K (2003) Up-regulation of soyasaponin biosynthesis by methyl jasmonate in cultured cells of Glycyrrhiza glabra. Plant Cell Physiol 44:404–411
Haralampidis K, Bryan G, Qi X, Papadopoulou K, Bakht S, Melton R, Osbourn A (2001) A new class of oxidosqualene cyclases directs synthesis of antimicrobial phytoprotectants in monocots. Proc Natl Acad Sci USA 98:13431–13436
Kang HJ, Anbazhagan VR, You XL, Moon HK, Yi JS, Choi YE (2006) Production of transgenic Aralia elata regenerated from Agrobacterium rhizogenes-mediated transformed roots. Plant Cell Tissue Organ Cult 85:187–196
Kartnig T, Hoffmann-Bohm K (1992) Centella. In: Hänsel R, Keller K, Rimpler H, Schneider G (eds) Hager’s handbuch der pharmazeutischen praxis. Springer, Berlin
Kim OT, Kim MY, Hong MH, Ahn JC, Hwang B (2004) Stimulation of asiaticoside production from Centella asiatica whole plant cultures by elicitors. Plant Cell Rep 23:339–344
Kim OT, Kim MY, Huh SM, Bai DG, Ahn JC, Hwang B (2005) Cloning of a cDNA probably encoding oxidosqualene cyclase associated with asiaticoside biosynthesis from Centella asiatica (L.) Urban. Plant Cell Rep 24:304–311
Komari T, Ishida Y, Hiei Y (2004) Plant transformation technology: Agrobacterium-mediated transformation. In: Christou P, Klee H (eds) Handbook of plant biotechnology. Wiley, England
Lee MH, Jeong JH, Seo JW, Shin CG, Kim YS, In JG, Yang DC, Yi JS, Choi YE (2004) Enhanced triterpene and phytosterol biosynthesis in Panax ginseng overexpressing squalene synthase gene. Plant Cell Physiol 45:976–984
Lu MB, Wong HL, Teng WL (2001) Effects of elicitation on the production of saponin in cell culture of Panax ginseng. Plant Cell Rep 20:647–677
Mangas S, Bonfill M, Osuna L, Moyano E, Tortoriello J, Cusido RM, Piñol MT, Palazón (2006) The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry 67:2041–2049
Matsuda H, Morikawa T, Ueda H, Yoshikawa M (2001) Medicinal foodstuffs. XXVII. Saponin constituents Gotu Kola (2): Structures of new ursane- and oleanane-type triterpene oligoglycosides, centellasaponin B, C, and D, from Centella asiatica cultivated in Sri Lanka. Chem Pharm Bull 49:1368–1371
Mihaljevic S, Stipkovic S, Jelaska S (1996) Increase of root induction in Pinus nigra explants using agrobacteria. Plant Cell Rep 15:610–614
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nath S, Buragohain AK (2005) Establishment of callus and cell suspension cultures of Centella asiatica. Biol Plant 49:411–413
Niu X, Li P, Veronese P, Bressan RA, Weller SC, Hasegawa PM (2000) Factors affecting Agrobacterium tumefaciens-mediated transformation of peppermint. Plant Cell Rep 19:304–310
Ottani MP, Schel JHN, Hänisch ten Cate Ch H (1990) Variation in structure and plant regeneration of Agrobacterium rhizogenes transformed and control roots of the potato cv. Bintje. Plant Cell Tissue Organ Cult 20:25–34
Paramageetham Ch, Prasad Babu G, Rao JVS (2004) Somatic embryogenesis in Centella asiatica L. an important medicinal and neutraceutical plant of India. Plant Cell Tissue Organ Cult 79:19–24
Patra A, Rai B, Rout GR, Das P (1998) Successful plant regeneration from callus culture of Centella asiatica (L.) Urban. Plant Growth Regul 24:13–16
Pointel JP, Boccalon H, Cloarec M, Ledebehat C, Joubert M (1987) Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology 38:46–50
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloing: a laboratory manual, 2ndedn. Cold Spring Harbor Laboratory Press, Plainview
Shi HP, Kintzios S (2003) Genetic transformation of Pueraria phaseoloides with Agrobacterium rhizogenes and puerarin production in hairy roots. Plant Cell Rep 21:1103–1107
Srivastava S, Srivastava AK (2007) Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol 27:29–43
Suzuki H, Achnine L, Xu R, Matsuda SPT, Dixon RA (2002) A genomic approach to the early stages of triterpene saponin biosynthesis in Medicago truncatula. Plant J 32:1033–1048
Tao J, Li L (2006) Genetic transformation of Torenia fournieri L. mediated by Agrobacterium rhizogenes. S Afr J Bot 72:211–216
Tiwari KN, Sharma NC, Tiwari V, Singh BD (2000) Micropropagation of Centella asiatica (L.), a valuable medicinal herb. Plant Cell Tissue Organ Cult 63:179–185
Acknowledgments
We thank Prof. Lourdes B. Cardenas, Institute of Biological Sciences, University of the Philippines Los Baños, for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. R. Liu.
Rights and permissions
About this article
Cite this article
Kim, OT., Bang, KH., Shin, YS. et al. Enhanced production of asiaticoside from hairy root cultures of Centella asiatica (L.) Urban elicited by methyl jasmonate. Plant Cell Rep 26, 1941–1949 (2007). https://doi.org/10.1007/s00299-007-0400-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0400-1