Abstract
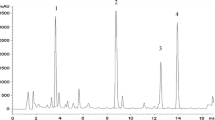
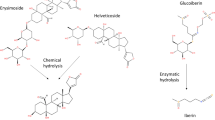
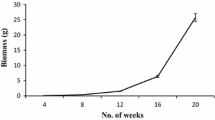
The localization was determined of the triterpenoids, asiaticoside and madecassoside, in different organs of glasshouse-grown plants and cultured material, including transformed roots, of two phenotypes of Centella asiatica (L.) Urban of Malaysian origin. Methanolic extracts of asiaticoside and madecassoside were prepared for gradient HPLC analysis. The two phenotypes of C. asiatica exhibited differences in terpenoid content that were tissue specific and varied between glasshouse-grown plants and tissue culture-derived material. Terpenoid content was highest in leaves, with asiaticoside (0.79 ± 0.03 and 1.15 ± 0.10 % of dry mass) and madecassoside [0.97 ± 0.06 and 1.65 ± 0.01 %(d.m.)] in the fringed (F) and smooth leaf (S) phenotypes, respectively. Roots of the F-phenotype contained the lowest content of asiaticoside [0.12 ± 0.01 %(d.m.)], whereas petioles of S-phenotype plants contained the lowest content of asiaticoside [0.16 ± 0.01 %(d.m.)] and madecassoside [0.18 ± 0.14 %(d.m.)]. Transformed roots were induced using Agrobacterium rhizogens and their growth was maximal on Murashige and Skoog basal medium supplemented with 60 g dm−3 sucrose. However, asiaticoside and madecassoside were undetectable in transformed roots and undifferentiated callus.
Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- BAP:
-
6-benzylaminopurine
- d.m.:
-
dry mass
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- ELISA:
-
enzyme-linked immunosorbent assay
- f.m.:
-
fresh mass
- IBA:
-
indolebutyric acid
- MS:
-
Murashige and Skoog (1962)
- LB:
-
Luria broth
- NAA:
-
α-naphthaleneacetic acid
- nptII:
-
neomycin phosphotransferase gene
- NPTII:
-
neomycin phosphotransferase protein
- SEM:
-
standard error of the mean
References
Al-Forkan, M., Power, J.B., Anthony, P., Lowe, K.C., Davey, M.R.: Agrobacterium-mediated transformation of Bangladeshi Indica rices.-Cell. mol. Biol. Lett. 9: 287–300, 2004.
Baek, Y.W.: Micropropagation of Centella asiatica (L.) Urban by In Vitro Cultures and Production of Triterpene Glycosides.-Ph.D. Thesis, Chonnan University, Gwangju 1997.
Balz, J.P., Courtois, D., Drieu, J., Drieu, K., Reynoird, J.P., Sohier, C., Teng, B.P., Touche, A., Petiard, V.: Production of ginkgolides and bilobalide by Ginkgo biloba plants and tissue cultures.-Planta med. 65: 620–626, 1999.
Banerjee, S., Zehra, M., Kumar, S.: In vitro multiplication of Centella asiatica, a medicinal herb from leaf explants.-Curr. Sci. 7: 147–148, 1999.
Booncong, P., Eshbaugh, W.H., Ogzewalla, C.D.: Centella asiatica (L.) (Apiaceae): Morphological and cytological characterization.-In: Nahrstedt, A. (ed.): Abstracts of Lectures and Poster Presentations in the 43rd Annual Congress on Medicinal Plant Research. Pp. 92–93. Halle 1995.
Cartayrade, A., Neau, E. Sohier, C., Balz, J.P., Carde, J.P., Walter, J.: Ginkgolide and bilobalide in Ginkgo biloba: 1. Sites of synthesis, translocation and accumulation of ginkgolides and bilobalide.-Plant Physiol. Biochem. 35: 859–868, 1997.
Collin, H.A.: Secondary product formation in plant tissue cultures.-Plant Growth Regul. 34: 119–134, 2001.
Curtis, I.S., Power, J.P., Blackhall, N.W., De Laat, A.M.M., Davey, M.R.: Genotype-independent transformation of lettuce using Agrobacterium tumefaciens.-J. exp. Bot. 45: 1441–1449, 1994.
Das, A., Mallick, R.: Correlation between genomic diversity and asiaticoside content in Centella asiatica (L.) Urban.-Bot. Bull. Acad. sin. 32: 1–8, 1991.
Dutta, T., Basu, U.P.: Isothankunic acid — a new triterpene acid from Centella asiatica (URB).-Bull. nat. Inst. Sci. India 37: 178–184, 1968.
Flück, H., Jaspersen-Schib, R.: Medicinal Plants and Their Uses.-Foulsham, New York 1976.
Giri, A., Narasu, M.L.: Transgenic hairy roots: recent trends and applications.-Biotechnol. Adv. 18: 1–22, 2000.
Granicher, F., Christen, P. Kamalaprija, P., Burger, U.: An iridoid diester from Valeriana officinalis var. sambucifolia hairy roots.-Phytochemistry 38: 103–105, 1995b.
Granicher, F., Christen, P., Kapetanidis, I.: Production of valepotriates by hairy root cultures of Centranthus ruber DC.-Plant Cell Rep. 14: 294–298, 1995a.
Gupta, A.P., Gupta, M.M., Kumar, S.: High performance thin layer chromatography of asiaticoside in Centella asiatica.-J. indian chem. Soc. 76: 321–322, 1999.
Inamdar, P.K., Yoele, R.D., Ghogare, A.B., De Souza, N.J.: Determination of biologically active constituents in Centella asiatica.-J. Chromatogr. A 742: 127–130, 1996.
Josekutty, P.C.: Callus culture and micropropagation of Hydrocotyle asiatica [Centella asiatica (L.) Urban], a medicinal plant.-Int. J. exp. Bot. 63: 275–278, 1998.
Kim, O.T., Kim, M.Y., Hong, M.H., Ahn, J.C., Hwang, B.: Stimulation of asiaticoside accumulation in the whole plant cultures of Centella asiatica (L.) Urban by elicitors.-Plant Cell Rep. 23: 339–344, 2004.
Kim, Y., Wyslouzil, B.E., Weathers, P.J.: Secondary metabolism of hairy root cultures in bioreactors.-In Vitro cell. dev. Biol. Plant 38: 1–10, 2002.
Mahagamasekera, M.G.P., Doran, P.M.: Intergeneric co-culture of genetically transformed organs for the production of scopolamine.-Phytochemistry 47: 17–25, 1998.
Massot, B., Milesi, S., Gontier, E., Bourgaud, F., Guckert, A.: Optimised culture conditions for the production of furanocoumarins by micropropagated shoots of Ruta graveolens.-Plant Cell Tissue Organ Cult. 62: 11–19, 2000.
Murashige, T., Skoog, F.: A revised medium for rapid growth and bioassays with tobacco tissue cultures.-Physiol. Plant. 15: 473–497, 1962.
Nath, S., Buragohain A.K.: Establishment of callus and cell suspension cultures of Centella asiatica.-Biol. Plant. 49: 411–414, 2005.
Opitz, S., Schnitzler, J.P., Hause, B., Schneider, B.: Histochemical analysis of phenylphenalenone-related compounds in Xiphidium caeruleum (Haemodoraceae).-Planta 216: 881–889, 2003.
Pythoud, F., Sinkar, V.P., Nester, E.W., Gordon, M.P.: Increased virulence of Agrobacterium rhizogenes conferred by the vir region of pTiBo542: Application to genetic engineering of poplar.-Bio/Technology 5: 1323–1327, 1987.
Rouillard-Guellec, F., Robin, J.R., Ratsimamanga, A.R., Ratsimamanga, S., Rasaoanaivo, P.: Comparative study of Centella asiatica of Madagascar origin and Indian origin.-Acta bot. gallica 144: 489–493, 1997.
Sambrook, J., Fritsch, E.F., Maniatis, T.: Molecular Cloning: A Laboratory Manual. 2nd Edition. Vol. 3.-Cold Spring Harbor Laboratory Press, New York 1989.
Samuelsson, G.: Drugs of Natural Origin, a Textbook of Pharmacognosy. Pp. 17–26. Swedish Pharmaceutical Press, Stockholm 1992.
Singh, B., Rastogi, R.P.: A reinvestigation of the triterpenes of Centella asiatica III.-Phytochemistry 8: 917–921, 1969.
Soldati, F.: Panax ginseng: standardization and biological activity.-In: Cutler, S.J., Cutler, H.G. (ed.): Biologically Active Natural Products: Pharmaceuticals. Pp. 209–232. CRC Press, Boca Raton 2000.
Solet, J.M., Bistermiel, F., Galons, H., Spagnoli, R., Guignard, J.L., Cosson, L.: Glucocosylation of thiocolchicine by a cell-suspension culture of Centella asiatica.-Phytochemistry 33: 817–820, 1993.
Snedecor, G.W., Cochran, W.G.: Statistical Methods. 8th Edition.-Iowa State Univ. Press, Ames 1989.
Subroto, A.M., Kwok, K.H., Hamill, J.D., Doran, P.M.: Co-culture of genetically transformed roots and shoots for synthesis, translocation and biotransformation of secondary metabolites.-Biotechnol. Bioeng. 49: 481–494, 1996.
Xu, T.F., Zhang, L., Sun, X.F., Zhang, H.M., Tang, K.X.: Production and analysis of organic acids in hairy-root cultures of Isatis indigotica Fort. (Indigo Woad).-Biotechnol. Appl. Biochem. 39: 123–128, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aziz, Z.A., Davey, M.R., Power, J.B. et al. Production of asiaticoside and madecassoside in Centella asiatica in vitro and in vivo . Biol Plant 51, 34–42 (2007). https://doi.org/10.1007/s10535-007-0008-x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10535-007-0008-x